Year: 2020

1 Doctoral position in the Vessel Formation in Development and Disease group
An ANR funded PhD position is available in the Vessel Formation in Development and Disease group at the iBV
The role of p16-dependent cellular senescence in healthy aging
Description :
Cellular senescence attracts attention as a key player contributing to organismal aging. The accumulation of senescent cells is dramatically increased with aging, however their precise contribution to aging-related phenotypes remains largely unclear. In collaboration with the team of D. Bulavin we showed p16-dependent senescent cells are required for healthy aging. We used different novel inducible mouse lines to characterise the role of p16 expressing cells in different organs. Currently, we focussed mainly on liver. The project aims at identifying the cell repertoire linked to aging-induced senescence and to investigate the impact of senescent cells on liver functions and to understand molecular pathways modulated by senescence. For these purposes we will use p16-Cre and p16-Cre-ERT2 mice crossed either with Rosa26-mTmG reporter or Rosa26-DTA ablator mice. The animals will be investigated by histological and immunohistological methods and RNA sequencing will be performed at different ages. This project will help to understand the molecular mechanism responsible for aging-induced activation of senescence and hopefully identify potential molecular targets to manipulate senescence through reprogramming and/or selective elimination of subsets of senescent cells.
Required Skills :
The working language is English.
Experience in molecular biology, cellular biology and/or mouse genetics would be a plus.
Motivation to work with mouse models and team orientation are required. Animal experimentation training is part of the project.
Related publications:
Grosse, L, Wagner, N, Emelyanov, A, Molina, C, Lacas-Gervais, S, Wagner, KD et al.. Defined p16High Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 2020:. doi: 10.1016/j.cmet.2020.05.002. PubMed PMID:32485135 .
Wagner, KD, Du, S, Martin, L, Leccia, N, Michiels, JF, Wagner, N et al.. Vascular PPARβ/δ Promotes Tumor Angiogenesis and Progression. Cells. 2019;8 (12):. doi: 10.3390/cells8121623. PubMed PMID:31842402 PubMed Central PMC6952835.
Wagner, KD, El Maï, M, Ladomery, M, Belali, T, Leccia, N, Michiels, JF et al.. Altered VEGF Splicing Isoform Balance in Tumor Endothelium Involves Activation of Splicing Factors Srpk1 and Srsf1 by the Wilms' Tumor Suppressor Wt1. Cells. 2019;8 (1):. doi: 10.3390/cells8010041. PubMed PMID:30641926 PubMed Central PMC6356959.
Wagner, KD, Ying, Y, Leong, W, Jiang, J, Hu, X, Chen, Y et al.. The differential spatiotemporal expression pattern of shelterin genes throughout lifespan. Aging (Albany NY). 2017;9 (4):1219-1232. doi: 10.18632/aging.101223. PubMed PMID:28437249 PubMed Central PMC5425123.
Wagner, KD, Cherfils-Vicini, J, Hosen, N, Hohenstein, P, Gilson, E, Hastie, ND et al.. The Wilms' tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun. 2014;5 :5852. doi: 10.1038/ncomms6852. PubMed PMID:25510679 .
El Maï, M, Wagner, KD, Michiels, JF, Ambrosetti, D, Borderie, A, Destree, S et al.. The Telomeric Protein TRF2 Regulates Angiogenesis by Binding and Activating the PDGFRβ Promoter. Cell Rep. 2014;9 (3):1047-60. doi: 10.1016/j.celrep.2014.09.038. PubMed PMID:25437559 .
Contacts:
Kay-Dietrich Wagner – kwagner@unice.fr
Nicole Wagner – nwagner@unice.fr
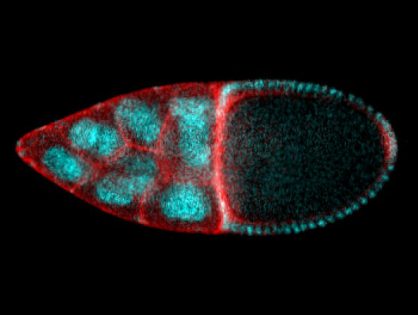
The egg puts on a corset to get the right shape
Thanks to an interdisciplinary and exciting collaboration between the iBV and the CBI in Toulouse, the Rauzi and the Wang teams unveiled a Cdc42 dependent mechanical process responsible for tissue elongation in the developing Drosophila egg chamber. By engineering new optogenetic techniques and infra-red laser based surgery, Anna Popkova and others show that a polarized supracellular actomyosin network, working as a molecular corset, generates tissue scale forces directing egg chamber elongation. Finally, this study opens new avenues to better understand how supra-cellular cytoskeletal networks emerge to drive embryo-scale morphogenesis during development. This work was published in Nature Communications.
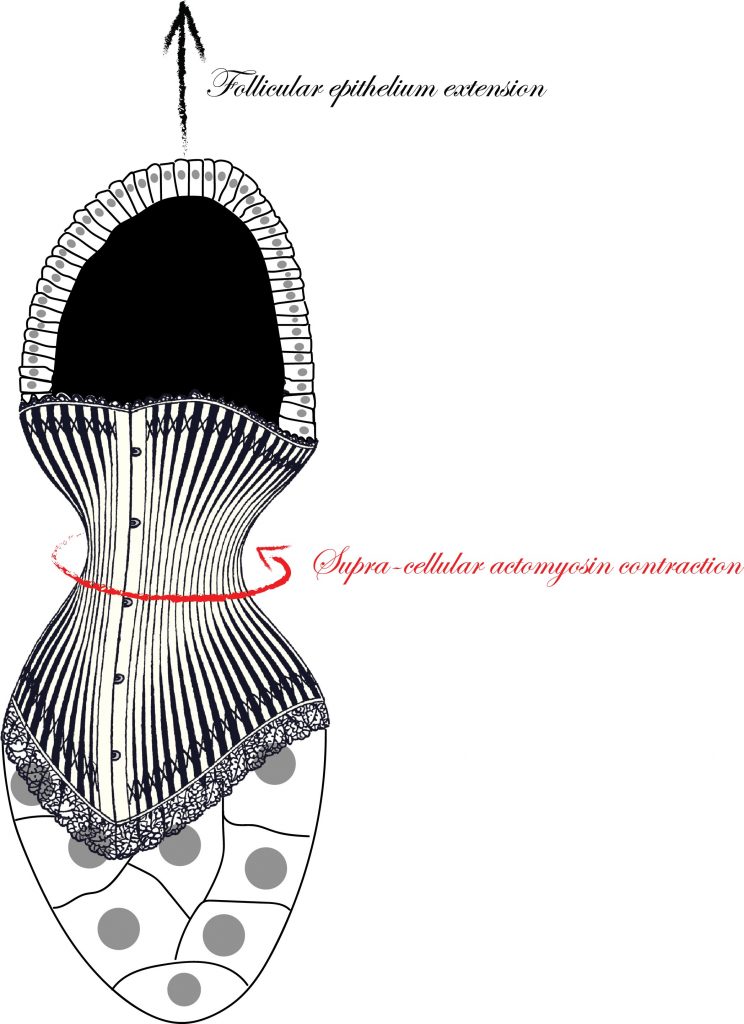
Understanding the mechanisms that are responsible to shape tissues and organs is an important and exciting challenge in developmental biology. The actomyosin cortex often plays a key role in generating the forces necessary to shape individual cells. Nevertheless, the actomyosin architecture can extend beyond the size of a single cell: cytoskeletal networks emerge at the scale of a tissue. These supra-cellular contractile structures can generate large forces that can rapidly shape epithelia.
In this study, scientists from the Rauzi and the Wang lab use the Drosophila egg chamber as a model system to study the origin and the mechanics of supra-cellular actomyosin networks. Popkova and others show that a supra-cellular array of parallel actin bundles, emerging from interdigitating filopodia, envelopes the follicle tissue. Filopodia radiate in a polarized way from basal stress fibers and extend by penetrating the neighboring cell cortexes. Filopodia can be mechanosensitive and function as anchors between cells. The small GTPase Cdc42 governs the formation of intercellular filopodia and stress fibers in the follicular cells. Thus, a Cdc42-dependent supracellular cytoskeletal network provides a scaffold integrating local oscillatory actomyosin contractions at the tissue scale to drive global polarized forces and tissue elongation.
Inset
Actin filaments assemble into diverse protrusive and contractile networks to generate forces in diverse cellular processes. Stress fibers are contractile higher order cytoskeletal structures composed of actomyosin bundles. Stress fibers play a key role in generating forces along the fiber direction and have important implication in cell adhesion to the extracellular matrix.
Filopodia are dynamic, finger-like plasma membrane protrusions that act as antennae to sense the mechanical and chemical environment. They are often regarded as “sensory organelles”. Filopodia are involved in many biological processes, such as growth cone guidance, cell migration, wound closure, and macrophage-induced cell invasion. These thin membrane protrusions are 60–200 nm in diameter and contain parallel bundles of 10–30 actin filaments held together by actin-binding proteins.
Laser dissection is a useful tool in developmental biology to probe the mechanical forces from the subcellular to the tissue/embryo scale. During tissue morphogenesis, cells are equipped with actomyosin networks generating forces. In this study, researchers used near-infrared (NIR) femtosecond (fs) pulsed laser surgery to dissect the actomyosin cytoskeleton with subcellular precision. This technique allows to selectively ablate actomyosin networks while preserving the cell plasma membrane. The resulting recoil of the remaining network, after laser dissection, is imaged and analyzed to deduce local forces responsible for tissue morphogenesis.
Optogenetics allows to control, via light stimulation, protein conformation changes and thus protein activity with spatial and temporal specificity. By using molecular engineering, proteins of interest are fused to photo-activatable proteins than can be expressed in specific cells. Using the optogenetic tool PA-Cdc42 which leads to the expression of a light-activatable Cdc42 protein, researchers were able to precisely determine the role of Cdc42 in follicular cells.
To read more:
A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension.
Popkova A, Stone OJ, Chen L, Qin X, Liu C, Liu J, Belguise K, Montell DJ, Hahn KM, Rauzi M@, Wang X@.
Nat Commun. 2020 Apr 21;11(1):1921. doi: 10.1038/s41467-020-15593-2.
Press release : Actualités scientifiques de l’INSB
Movie: Time-lapse of a representative mCD8GFP-expressing egg chamber labelled with MyoII-mCherry. Laser dissection of the supra-cellular actomyosin network was performed along the AP axis of the egg chamber. Scale bar 10 μm.

PhD Position - 3 years - Starting October 2020
Deciphering the regulation of the cell death receptor Fas by cell polarity molecules & adherens junctions in both tumoral and normal human epithelia
Key words: Receptor, Signaling, Cancer, Cell polarity, Cell-Cell jonctions
The PhD student will integrate the research group “death receptors signaling in cancer therapy” (iBV, http://ibv.unice.fr/research-team/hueber). at the Institute of Biology Valrose affiliated to the CNRS, Inserm and University Côte d’Azur (UCA).
Project proposal: Our team is investigating the functions of the cell death receptor Fas/CD95/TNFRSF6, a member of the TNFR superfamily. Fas is considered as a tumor suppressor thanks to its ability to eliminate cancer cells by engaging programmed cell death by apoptosis. However, Fas activation by its ligand (FasL) could also promote tumor development and immune disorders (1). Our group is studying the molecular mechanisms that control the Fas versatile signaling outcome in the context of both normal and cancer cells (2, 3). Our recent data show that formation of adherens junction, a cell-cell adhesion complex, and association with the Dlg1 polarity complex prevent the pro-apoptotic signaling of Fas (4, 5). This new Fas-regulatory mechanism is crucial to protect normal epithelial cells from apoptotic signals and to sense and eliminate abnormal cells from epithelial tissues to prevent pathological outcome such as cancer and chronic inflammatory diseases. The PhD student will pursue this project and decipher the regulation of Fas cell death and non- death signaling by the Cadherin-Dlg1 polarity complex notably by studying Fas receptor signaling/trafficking on both primary and tumoral epithelial human cells by using various cell biology approaches.
Bibliography of the team link to the project: 1- Rossin et al (2019) Cancers, 8;11(5):639. 2- NL t al (2018) Sci Rep, 20;8(1):12424. 3- Chakrabandhu K (2016), PLoS Biol, 4;14(3). 4- Gagnoux-Palacios, L.; et al (2018) Journal of Cell
Biology, 217, 3839-3852. 5- Gagnoux-Palacios L., Hueber AO (2019) Medecine/Sciences. 35(11):830-833.
Technical approaches: Human cell culture; cell death assays; receptor trafficking studies (cell surface labeling, endocytosis assay), protein expression quantification/localization (FACS, IP, IF, IB, ELISA, proteomic); microscopy techniques (confocal imaging, time-lapse).
Candidate profil: We are looking for a highly motivated student, independent and creative, with a Master’s Degree in Cellular, Cancer or Molecular Biology. Prior experience with cell culture and classical cellular and biochemical approaches will be appreciated.
HOW TO APPLY: Interested and motivated students should send as soon as possible a CV, a motivation letter, master scores/ranking and reference letters to both L. GAGNOUX (gagnoux@unice.fr) and A-O HUEBER (hueber@unice.fr).

1 Doctoral position - Deadline June 1st, 2020 (Optogenetics)
Doctoral position at University Côte d’Azur: Fungal Cell Biology
Light-dependent regulation of cell polarity in a fungal pathogen
The fungus Candida albicans is normally a harmless commensal that is found on mucosal surfaces of the gastrointestinal and urogenital tract in most healthy individuals. This commensal organism can cause superficial as well as life-threatening systemic infections in response to alterations of its environment, and is particularly aggressive in immuno-compromised individuals. As an opportunistic pathogen it can colonize and infect different body sites and is responsible for one of the most predominant fungal nosocomial infections. The ability of this fungus to switch from an ovoid form to a filamentous form is critical for its pathogenicity, in particularly its ability to invade and penetrate into host tissues and evade and burst out of host immune cells.
The recent advent of light-dependent approaches to control protein subcellular localization has made possible the specific alteration of growth, circumventing classical genetic and chemical perturbations. We have optimized such a light-dependent protein targeting system for C. albicans, which gives us exquisite control of growth in this organism. In addition, new variants of these systems have been established which facilitate their use and response time. Furthermore, a number of new, spectrally distinct fluorescent proteins, which we have optimized for use in C. albicans, now make it possible to follow different cellular processes simultaneously during light-dependent perturbation of growth and cell polarity. The goal of this project is to use such a light-dependent protein targeting to probe filamentous growth as well as invasive filamentous growth in a human fungal pathogen. The project will use a combination of molecular biology, microbiology and live cell microscopy to probe filamentous growth and morphogenesis in this fungal human pathogen.
We are seeking highly motivated candidates with a background in Cell Biology and interest in live cell imaging. Experience in Microbiology would be a plus.
Interested candidates can contact R. Arkowitz (arkowitz@unice.fr) by June 1st
1) M Bassilana, C Puerner & RA Arkowitz. Curr. Opin. Cell Biol. 2020 62:150-158.
2) PM Silva, C Puerner, A Seminara, M Bassilana & RA Arkowitz. Cell Rep. 2019 28:2231–2245.
3) RA Arkowitz & M Bassilana. F1000 Res. 2019 8.
4) A Weiner, F Orange, S Lacas-Gervais, K Rechav, V Ghugtyal, M Bassilana & RA Arkowitz. Cell Microbiol. 2019 21: e12963
5) H Labbaoui, S Bogliolo, V Ghugtyal, NV Solis, SG Filler, RA Arkowitz & M Bassilana. Plos Pathog. 2017 13: e1006205