
Ellen VAN OBBERGHEN-SCHILLING
Adhesion Signaling and Stromal Reprogramming in the Tumor Microenvironment
Main interests
- Understanding mechanisms regulating the expression and alternative splicing of oncofetal ECM proteins and how the presence of alternatively-spliced domains impacts their assembly and functions
- Elucidating how the tumor ECM enhances the invasive behavior of tumor cells, promotes the angiogenic phenotype of endothelial cells and blocks the anti-tumor functions of immune cells
- Obtaining clinically relevant knowledge about how the neoplastic ECM contributes to stromal immunosuppressive programs in human head and neck carcinomas
Scientific Questions
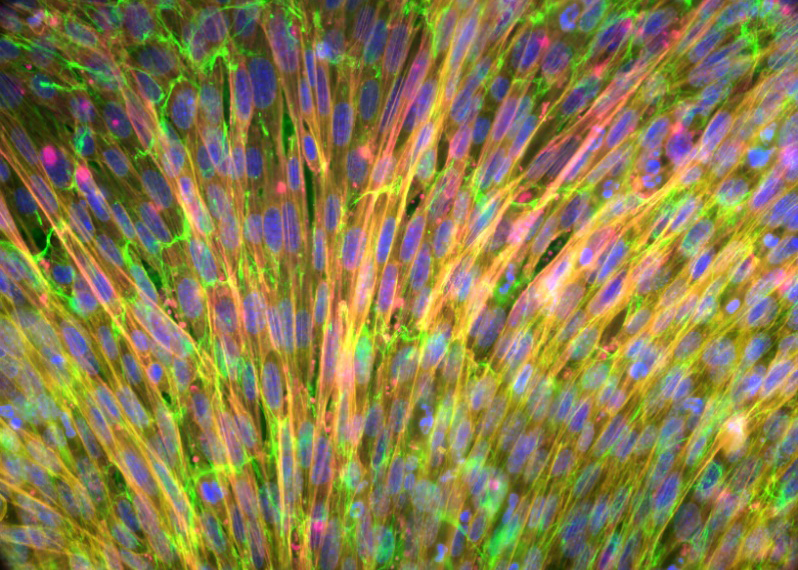
Our research focuses on the role of the extracellular matrix (ECM) in carcinoma progression, spread and response to therapy.
For a tumor to develop and expand, the growth-repressive environment of the host tissue must undergo significant changes. These changes, brought about through cancer cell-stromal interactions, include dramatic modifications in the molecular composition and architecture of the ECM. Re-expression of “oncofetal” matrix proteins, known to play determinant roles in tissue morphogenesis during embryonic development, participates in the pro-tumoral conversion of the stroma through complex signaling networks and biomechanical mechanisms that are not well understood. Our laboratory seeks to uncover mechanisms underlying tumor-induced stromagenic reprogramming and to elucidate how the neoplastic ECM enhances the invasive behavior of tumor cells, escape from immune surveillance systems and therapeutic resistance.
Our Strategy
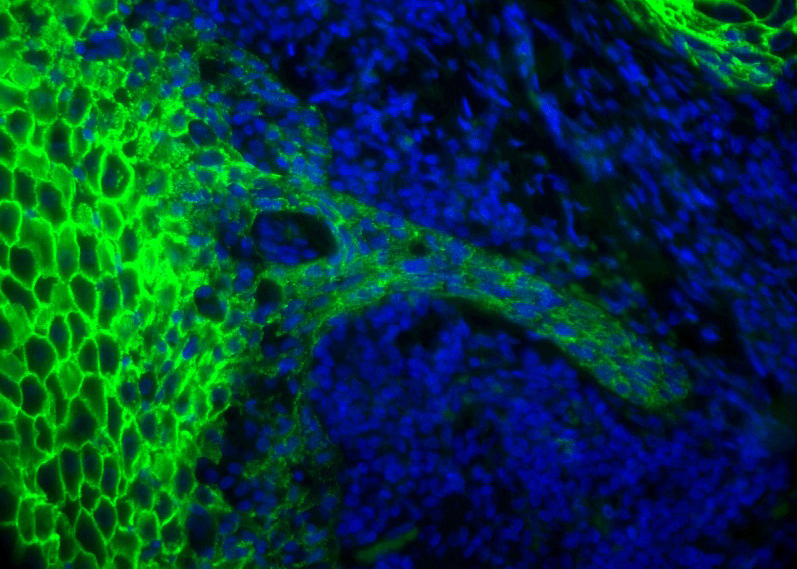
Efforts of the team are centered on head and neck squamous cell carcinoma (HNSCC), a tumor pathology for which we have established in vitro, ex vivo and in vivo experimental models through a collaborative network of basic and clinical research teams.
Our characterization of the ECM landscape of human HNSCC-associated fibroblasts allowed us to identify oncofetal fibronectin (FN) and 2 ECM receptors on carcinoma cells as key molecular determinants of the tumor-stroma dialog in HNSCC. Current projects aim: (i) to understand mechanisms regulating the expression of oncofetal ECM proteins and how the presence of alternatively spliced domains impacts the assembly/nanotopology and functions of the proteins, (ii) to elucidate how oncofetal components of the neoplastic ECM contribute to stromal immunosuppressive programs, and iii) to identify ECM-associated therapeutic targets and predictive biomarkers for improved efficacy of emerging treatments.
These aims are addressed through a complementary set of cellular, molecular and computational approaches involving the use of 3D culture models, full length recombinant matrix proteins and analysis of patient samples in collaboration with our clinical colleagues.
Research Aims
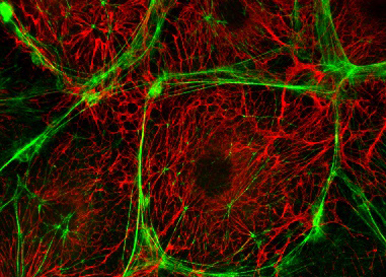
Understanding mechanisms regulating the expression and alternative splicing of oncofetal ECM proteins and how the presence of alternatively-spliced domains impacts their assembly and functions
Oncofetal FN differs from plasma FN by the presence of highly conserved “Extra Domains” that increase the repertoire of cellular receptors and alter the function of the protein in ways that we are investigating using an isoform-specific biological toolset and an interdisciplinary approach.
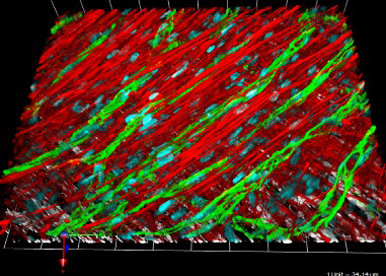
Elucidating how the neoplastic ECM contributes to the progression and spread of head and neck carcinomas
Using a variety of approaches including 3D culture systems and analysis of human tumors, and based on insights from a carcinogen-induced model of oral SCC in immune-competent mice (collaborators), we aim to understand how the neoplastic ECM promotes the invasive behavior of tumor cells and angiogenic phenotype of endothelial cells, and blocks tumor-suppressive functions of immune cells.
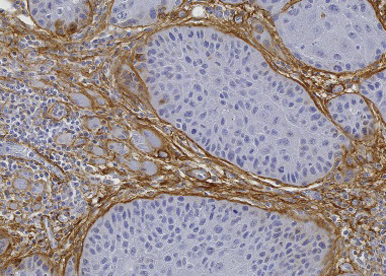
ECM reprogramming and anti-tumor immunity in human tumors
Immunomodulatory therapies are promising for HNSCC yet only a fraction patients respond. Thus offering better responses to these treatments is an urgent and unmet clinical need. To further understand ECM-regulated control of anti-tumor immunity in human tumors, and provide a foundation for clinically relevant discoveries, we are participating in biological studies linked to immunotherapy trials in different therapeutic settings.
Clinical Researchers
 SUDAKA Anne - +33 489150790
SUDAKA Anne - +33 489150790 PEYRADE Frédéric - +33 489150790
PEYRADE Frédéric - +33 489150790 SAADA-BOUZID Esma - +33 492031514
SAADA-BOUZID Esma - +33 492031514
Recent Publications
- Grapa, AI, Efthymiou, G, Van Obberghen-Schilling, E, Blanc-Féraud, L, Descombes, X. A spatial statistical framework for the parametric study of fiber networks: Application to fibronectin deposition by normal and activated fibroblasts. Biol Imaging. 2023;3 :e25. doi: 10.1017/S2633903X23000247. PubMed PMID:38510171 PubMed Central PMC10951922.
- Rekad, Z, Ruff, M, Radwanska, A, Grall, D, Ciais, D, Van Obberghen-Schilling, E et al.. Coalescent RNA-localizing and transcriptional activities of SAM68 modulate adhesion and subendothelial basement membrane assembly. Elife. 2023;12 :. doi: 10.7554/eLife.85165. PubMed PMID:37585334 PubMed Central PMC10431919.
- Tunali, G, Yanik, H, Ozturk, SC, Demirkol-Canli, S, Efthymiou, G, Yilmaz, KB et al.. A positive feedback loop driven by fibronectin and IL-1β sustains the inflammatory microenvironment in breast cancer. Breast Cancer Res. 2023;25 (1):27. doi: 10.1186/s13058-023-01629-0. PubMed PMID:36922898 PubMed Central PMC10015813.
- Rekad, Z, Izzi, V, Lamba, R, Ciais, D, Van Obberghen-Schilling, E. The alternative matrisome: Alternative splicing of ECM proteins in development, homeostasis and tumor progression. Matrix Biol. 2022;111 :26-52. doi: 10.1016/j.matbio.2022.05.003. PubMed PMID:35537652 .
- Elaldi, R, Hemon, P, Petti, L, Cosson, E, Desrues, B, Sudaka, A et al.. High Dimensional Imaging Mass Cytometry Panel to Visualize the Tumor Immune Microenvironment Contexture. Front Immunol. 2021;12 :666233. doi: 10.3389/fimmu.2021.666233. PubMed PMID:33936105 PubMed Central PMC8085494.
- Saint, A, Van Obberghen-Schilling, E. The role of the tumor matrix environment in progression of head and neck cancer. Curr Opin Oncol. 2021;33 (3):168-174. doi: 10.1097/CCO.0000000000000730. PubMed PMID:33720067 .
- Efthymiou, G, Radwanska, A, Grapa, AI, Beghelli-de la Forest Divonne, S, Grall, D, Schaub, S et al.. Fibronectin Extra Domains tune cellular responses and confer topographically distinct features to fibril networks. J Cell Sci. 2021;134 (4):. doi: 10.1242/jcs.252957. PubMed PMID:33526715 .
- Spenlé, C, Loustau, T, Murdamoothoo, D, Erne, W, Beghelli-de la Forest Divonne, S, Veber, R et al.. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol Res. 2020;8 (9):1122-1138. doi: 10.1158/2326-6066.CIR-20-0074. PubMed PMID:32665262 .
- Efthymiou, G, Saint, A, Ruff, M, Rekad, Z, Ciais, D, Van Obberghen-Schilling, E et al.. Shaping Up the Tumor Microenvironment With Cellular Fibronectin. Front Oncol. 2020;10 :641. doi: 10.3389/fonc.2020.00641. PubMed PMID:32426283 PubMed Central PMC7203475.
- Hofman, P, Ayache, N, Barbry, P, Barlaud, M, Bel, A, Blancou, P et al.. The OncoAge Consortium: Linking Aging and Oncology from Bench to Bedside and Back Again. Cancers (Basel). 2019;11 (2):. doi: 10.3390/cancers11020250. PubMed PMID:30795607 PubMed Central PMC6406685.
- Soubies, E, Radwanska, A, Grall, D, Blanc-Féraud, L, Van Obberghen-Schilling, E, Schaub, S et al.. Nanometric axial resolution of fibronectin assembly units achieved with an efficient reconstruction approach for multi-angle-TIRF microscopy. Sci Rep. 2019;9 (1):1926. doi: 10.1038/s41598-018-36119-3. PubMed PMID:30760745 PubMed Central PMC6374485.
- Radwanska, A, Grall, D, Schaub, S, Divonne, SBF, Ciais, D, Rekima, S et al.. Counterbalancing anti-adhesive effects of Tenascin-C through fibronectin expression in endothelial cells. Sci Rep. 2017;7 (1):12762. doi: 10.1038/s41598-017-13008-9. PubMed PMID:28986537 PubMed Central PMC5630602.
- Degli Esposti, D, Sklias, A, Lima, SC, Beghelli-de la Forest Divonne, S, Cahais, V, Fernandez-Jimenez, N et al.. Unique DNA methylation signature in HPV-positive head and neck squamous cell carcinomas. Genome Med. 2017;9 (1):33. doi: 10.1186/s13073-017-0419-z. PubMed PMID:28381277 PubMed Central PMC5382363.
- Gopal, S, Veracini, L, Grall, D, Butori, C, Schaub, S, Audebert, S et al.. Fibronectin-guided migration of carcinoma collectives. Nat Commun. 2017;8 :14105. doi: 10.1038/ncomms14105. PubMed PMID:28102238 PubMed Central PMC5253696.
- Rupp, T, Langlois, B, Koczorowska, MM, Radwanska, A, Sun, Z, Hussenet, T et al.. Tenascin-C Orchestrates Glioblastoma Angiogenesis by Modulation of Pro- and Anti-angiogenic Signaling. Cell Rep. 2016;17 (10):2607-2619. doi: 10.1016/j.celrep.2016.11.012. PubMed PMID:27926865 .
- Sakakini, N, Turchi, L, Bergon, A, Holota, H, Rekima, S, Lopez, F et al.. A Positive Feed-forward Loop Associating EGR1 and PDGFA Promotes Proliferation and Self-renewal in Glioblastoma Stem Cells. J Biol Chem. 2016;291 (20):10684-99. doi: 10.1074/jbc.M116.720698. PubMed PMID:27002148 PubMed Central PMC4865916.
- Thariat, J, Vignot, S, Lapierre, A, Falk, AT, Guigay, J, Van Obberghen-Schilling, E et al.. Integrating genomics in head and neck cancer treatment: Promises and pitfalls. Crit Rev Oncol Hematol. 2015;95 (3):397-406. doi: 10.1016/j.critrevonc.2015.03.005. PubMed PMID:25979769 .
- Veracini, L, Grall, D, Schaub, S, Beghelli-de la Forest Divonne, S, Etienne-Grimaldi, MC, Milano, G et al.. Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas. Oncotarget. 2015;6 (10):7570-83. doi: 10.18632/oncotarget.3071. PubMed PMID:25779657 PubMed Central PMC4480700.
- Turchi, L, Debruyne, DN, Almairac, F, Virolle, V, Fareh, M, Neirijnck, Y et al.. Tumorigenic potential of miR-18A* in glioma initiating cells requires NOTCH-1 signaling. Stem Cells. 2013;31 (7):1252-65. doi: 10.1002/stem.1373. PubMed PMID:23533157 .
- Thariat, J, Bensadoun, RJ, Etienne-Grimaldi, MC, Grall, D, Penault-Llorca, F, Dassonville, O et al.. Contrasted outcomes to gefitinib on tumoral IGF1R expression in head and neck cancer patients receiving postoperative chemoradiation (GORTEC trial 2004-02). Clin Cancer Res. 2012;18 (18):5123-33. doi: 10.1158/1078-0432.CCR-12-1518. PubMed PMID:22855581 .

Research assistant (multiplex fluorescence immunohistochemistry) in Nice, France
Job description
We are seeking a very motivated and highly organized research assistant to join our multidisciplinary team of basic and clinical researchers. The candidate will be primarily responsible for the management and multi-color immunofluorescence staining of FFPE tumor samples from head and neck cancer patients enrolled in a clinical immunotherapy trial. The goal of this study is to identify biomarkers from the immune and extracellular matrix microenvironment that are predictive of treatment response.
Principal duties and responsibilities
- Conduct multi-color fluorescence immunohistochemistry on human tissue samples
- Scan stained slides and perform spectral unmixing for image analysis
- Manage slides and digital images
- Maintain detailed documentation of experimental work
- Participate in the preparation of documents for presentation/publication
- Assist in purchasing, maintenance and organizational tasks in the laboratory
Required Skills
- Scientific understanding of, and experience in, histological techniques and immunofluorescence staining of tissue
- Excellent organizational skills and attention to details
- Proficiency in utilizing spreadsheet software
- Strong communication and interpersonal skills
- Good knowledge of written and spoken English
Working environment and location
The candidate will be involved in a challenging project funded by the Fondation ARC (French Cancer Research Association) that is dedicated to the search for tumor microenvironmental biomarkers predictive of a response to immunotherapy. This project brings together biologists, immunologists, pathologists and clinicians from Nice, Paris and Lyon. The activity will be carried out in the team “Matrix microenvironment and tumor progression” (E. Van Obberghen- Schilling) at the Institute of Biology Valrose (iBV, Nice, France).
Opportunity for professional development
Gain experience in translational research, networking in a collaborative multi-centric/multi-disciplinary project, cutting- edge immunohistochemistry techniques and digital pathology.
Duration : 14 months
Starting Date : October 15, 2021
Salary : Based on the civil service grid, according to experience (CNRS, Level AI)
Contact and information:
Ellen Van Obberghen-Schilling (vanobber@unice.fr)
Equipe Microenvironnement matriciel et progression tumorale Institute of Biology Valrose (iBV)
Parc Valrose, Université Côte d’Azur 06108 Nice, France

Translating architecture of the tumor extracellular matrix into function
3IA Côte d’Azur Research Axis : AI for Computational Biology and Bio-Inspired AI Applications are invited for a 2-year 3iA Côte d’Azur postdoctoral position in tumor/ECM biology to study functional and structural features of the extracellular matrix (ECM) in the immunosuppressive tumor microenvironment of head and neck cancer. Immunomodulatory therapies are promising for this tumor type, yet resistance rates are high since less than 20% of patients respond. We are specifically interested in exploring the tumor ECM environment, together with immune cell signatures, for gaining mechanistic insights into invasive disease and resistance to immunotherapy. Our previous work on ECM topology has provided a framework for quantitative description and modeling of matrix features associated with disease states. The present project involves functional analysis of matrix components in cell culture models, and quantitative characterization of ECM architecture in human tumor tissue using multiplex immunofluorescence imaging.
Biological studies will be carried out in the Institute of Biology Valrose, a leading Research Center of the Université Côte d’Azur (UCA) equipped with state of the art core facilities and a dynamic and international scientific environment. Computational analyses will be performed in close collaboration with biological image processing experts of the I3S Laboratory, UCA (MORPHEM group) and clinical partners, thus providing interdisciplinary training at the crossroads of ECM biology, computational biology and cancer research.
Profile: The candidate is expected to be a creative, quantitatively-minded biologist (PhD or MD PhD) with a strong background in cell/tissue imaging. Previous experience in tumor biology and the ECM are an advantage. Excellent communication skills, both written and oral, are required.
Please send applications (motivation statement, full CV, 2 letters of recommendation including one from PhD thesis advisor) to: Ellen Van Obberghen-Schilling (vanobber@unice.fr). Further information is available on the 3iA Côte d’Azur website.
Selected recent publications of the host team:
- Spenlé C et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol Res. 2020 Sep;8(9):1122-1138.
- Efthymiou G et al. Shaping Up the Tumor Microenvironment with Cellular Fibronectin. Front Oncol. 2020 Apr 30;10:641.
- Efthymiou G et al. Fibronectin Extra Domains tune cellular responses and confer topographically distinct features to fibril networks. Journal of Cell Science 2021 : jcs.252957 doi: 10.1242/jcs.252957

AI-based analysis of the tumor extracellular matrix
3IA Côte d’Azur Research Axis : AI for Computational Biology and Bio-Inspired AI Applications are invited for a 2-year 3iA Côte d’Azur postdoctoral position in tumor/ECM biology to study functional and structural features of the extracellular matrix (ECM) in the immunosuppressive tumor microenvironment of head and neck cancer. Immunomodulatory therapies are promising for this tumor type, yet resistance rates are high since less than 20% of patients respond. We are specifically interested in exploring the tumor ECM environment, together with immune cell signatures, for gaining mechanistic insights into invasive disease and resistance to immunotherapy. Our previous work on ECM topology based on confocal images of cell-derived ECM has provided a framework for quantitative description and modeling of matrix features associated with disease states. The present project involves quantitative characterization of ECM architecture in in vitro models and human tumor tissue using multiplex immunofluorescence imaging, empowered by deep learning approaches.
Biological studies will be carried out in the Institute of Biology Valrose, a leading Research Center of the Université Côte d’Azur (UCA) equipped with state of the art core facilities and a dynamic scientific environment. Computational analyses will be performed in close collaboration with biological image processing experts of the I3S Laboratory, UCA (MORPHEM group) and clinical partners, thus providing interdisciplinary training at the crossroads of ECM/tumor biology, computational biology and human tumor pathology.
Profile: The candidate should be a creative, quantitatively-minded biologist with a strong background in cell/tissue imaging. Previous experience in tumor biology and the ECM are an advantage. Excellent communication skills, both written and oral (English), are required.
Please send applications (motivation statement, full CV, 2 letters of recommendation including one from PhD thesis advisor) to : Ellen Van Obberghen-Schilling. Further information is available on the 3IA Côte d’Azur website.
Selected recent publications of the host team:
Spenlé C et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol Res. 2020 Sep;8(9):1122-1138.
Efthymiou G et al. Shaping Up the Tumor Microenvironment with Cellular Fibronectin. Front Oncol. 2020 Apr 30;10:641.
Efthymiou G et al. Fibronectin Extra Domains tune cellular responses and confer topographically distinct features to fibril networks. Journal of Cell Science 2021 : jcs.252957 doi: 10.1242/jcs.252957
2 Postdoctoral Research Positions
in Nice France
To study the impact of the tumor matrix environment on immune cells
Applications are invited for 2 full-time postdoctoral positions in tumor/immuno-biology to study the implication of extracellular matrix (ECM) components in the immunosuppressive reprogramming of the tumor microenvironment in head and neck cancer associated with tobacco use. The project aims to provide a better understanding of the cellular, molecular and functional interactions between tumor-associated innate immune cells and ECM molecules during cancerogenesis of head and neck squamous epithelium. Studies will be conducted using an immunocompetent mouse model of tongue carcinogenesis and ex vivo 3D reconstituted ECM systems. Implications of our findings for human cancer and therapeutic intervention will be determined by analyzing samples from human patients. This collaborative project between teams in Nice and Strasbourg will provide training in an interdisciplinary context at the crossroads of immunology, cell and molecular biology and human tumor pathology.
One position is available in the Institute of Biology Valrose (iBV) (http://ibv.unice.fr) and one position in the Institute of Molecular and Cellular Pharmacology (IPMC) (https://www.ipmc.cnrs.fr/cgi-bin/ipmcx.cgi). These leading Research Centers of the Université Côte d’Azur are equipped with state of the art core facilities and dynamic scientific environments in which English is the working language. The iBV team headed by E. Van Obberghen-Schilling has an expertise cell-matrix adhesion and matrix-driven signaling in squamous cell carcinomas of the head and neck. F. Anjuère, leader of the IPMC team “Immune regulations at muco-cutaneous surfaces” with V. Braud, is an expert in mucosal immunology and has led numerous studies aimed at developing mucosal vaccines against infectious diseases and cancer. Clinical collaborators at the head and neck oncology team of the Centre Antoine Lacassagne (CAL) and University Head and Neck Institute (IUFC) are actively involved in clinical studies focused on immunotherapy.
The successful post-doctoral candidates should have a strong background in cell biology/immunology and experience in cellular/tissue imaging. Excellent written and spoken English communication skills, strong self-motivation, the ability to work both independently and collaboratively is expected.
Please send applications (full CV including research interests and the name of 2-3 referees in a single pdf document) and requests for further information by email to:
Ellen Van Obberghen-Schilling (vanobber@unice.fr) – Fabienne Anjuère (anjuere@ipmc.cnrs.fr)
Starting date: November 2017
2001 - Research Award in Physiology/Pathology, Inserm
1996 - Young Scientist Award, FRM
1991 - Prix Exceptionnel, Groupe d'Etudes sur l'Hémostase et la Thrombose

Immune cells held hostage in the tumor stroma!
Read More
“Multi-angle-TIRF” : a molecular resolution optical microscope developed at iBV
Read More

UCA annual Award Ceremony: 7 iBV members recognised for their scientific contributions
Read More

Poster Prize awarded to UCA PhD student in International Conference, Berlin 2018
Read More
iBV - Institut de Biologie Valrose
"Sciences Naturelles"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
