
Kay WAGNER
Vessel formation in development and disease
Main interests
- cardiovascular development
- regeneration
- cancer and tumour stroma cell interaction
- angiogenesis
Scientific Questions
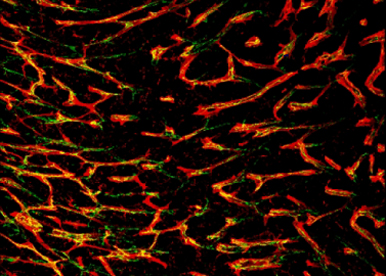
The primary interest of our research is to understand how vessels grow and re-model. As besides endothelial cells, different cell types, e.g. hematopoietic progenitor and myeloid derived cells, pericytes, and smooth muscle cells are involved in vessel formation and maturation, we are interested in the contribution of these different cell types for vessel formation under physiological and pathological conditions. Furthermore, we want to identify signals from surrounding tissues, e.g. myocardium, tumour stroma and immune cells, which modify vessel growth. Finally, the aim of our projects is to translate our findings into potential novel therapeutic approaches and to identify new pharmacological candidates to modify vascular growth.
Our Strategy
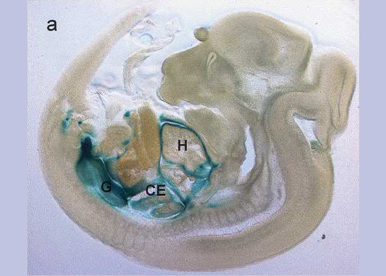
The cardiovascular system is the first functional organ system required for embryonic survival. Many major diseases and pathophysiological processes are associated with vessel formation, e.g. coronary artery disease, peripheral arterial diseases, liver fibrosis, wound healing, graft rejection, or cancer. Chronic ischemic heart disease, myocardial infarction, and cancer are the most common causes for morbidity and mortality. In ischemic heart disease, angiogenesis is required for collateral vessel formation and therefore a “friend”, in cancer it becomes a “foe” by inducing the transition of a tumour from a small cluster of malignant cells to a macroscopic tumour capable of spreading to other organs via the vasculature.
We use mainly analyses in mice in vivo (reporter and lineage tracing strains, inducible knockout / overexpression / knockin mice).
The in vivo data are supported by cell culture systems and molecular approaches. We use CRISPR/Cas9 technology to generate reporter cell lines, which serve to test small molecule libraries for their potential to inhibit / activate genes of interest.
Research Aims
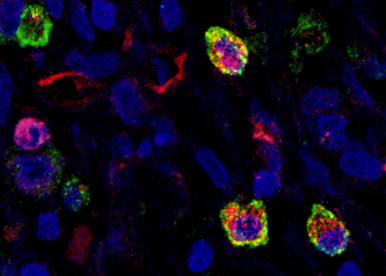
Characterization of different cell types contributing to tumour angiogenesis and progression.

Identification of proteins critically involved in cardiac angiogenesis and repair.
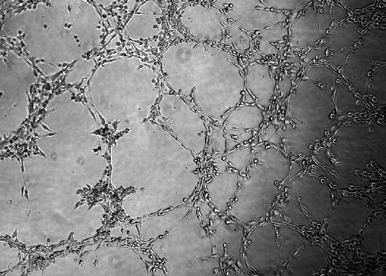
Discovery of new small molecules to modify vascular formation.
Researchers
 WAGNER Nicole - +33 489153713
WAGNER Nicole - +33 489153713
Clinical Researchers
 LECCIA Nathalie - +33 493377665
LECCIA Nathalie - +33 493377665 CHAU Yves - +33 A
CHAU Yves - +33 A
Postdocs
 DIAZ DEL MORAL Sandra - +33 R
DIAZ DEL MORAL Sandra - +33 R
Students
 DENIS Ornella - +33 R
DENIS Ornella - +33 R
- Wagner, N, Vukolic, A, Baudouy, D, Pagnotta, S, Michiels, JF, Wagner, KD et al.. Enhanced neoangiogenesis and balance of the immune response mediated by the Wilms' tumor suppressor WT1 favor repair after myocardial infarction. Theranostics. 2025;15 (14):6593-6614. doi: 10.7150/thno.104329. PubMed PMID:40585983 PubMed Central PMC12203559.
- Díaz Del Moral, S, Wagner, N, Wagner, KD. The Wilms' Tumor Suppressor WT1 in Cardiomyocytes: Implications for Cardiac Homeostasis and Repair. Cells. 2024;13 (24):. doi: 10.3390/cells13242078. PubMed PMID:39768169 PubMed Central PMC11674098.
- Wagner, KD, Safwan-Zaiter, H, Wagner, N. A Dual Role of the Senescence Marker P16Ink4a in Liver Endothelial Cell Function. Cells. 2024;13 (23):. doi: 10.3390/cells13231929. PubMed PMID:39682678 PubMed Central PMC11640762.
- Díaz Del Moral, S, Benaouicha, M, Villa Del Campo, C, Torres, M, Wagner, N, Wagner, KD et al.. Correction: Díaz del Moral et al. Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage. J. Cardiovasc. Dev. Dis. 2023, 10, 211. J Cardiovasc Dev Dis. 2024;11 (7):. doi: 10.3390/jcdd11070190. PubMed PMID:39057649 PubMed Central PMC11256256.
- Wagner, N, Wagner, KD. Recent Insights into the Role of PPARs in Disease. Cells. 2023;12 (12):. doi: 10.3390/cells12121572. PubMed PMID:37371042 PubMed Central PMC10296837.
- Wagner, N, Wagner, KD. Molecular Mechanisms of Cardiac Development and Disease. Int J Mol Sci. 2023;24 (10):. doi: 10.3390/ijms24108784. PubMed PMID:37240127 PubMed Central PMC10218175.
- Díaz Del Moral, S, Benaouicha, M, Villa Del Campo, C, Torres, M, Wagner, N, Wagner, KD et al.. Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage. J Cardiovasc Dev Dis. 2023;10 (5):. doi: 10.3390/jcdd10050211. PubMed PMID:37233178 PubMed Central PMC10219250.
- Wagner, N, Wagner, KD. Controversies and Recent Advances in Senescence and Aging. Cells. 2023;12 (6):. doi: 10.3390/cells12060902. PubMed PMID:36980243 PubMed Central PMC10046983.
- Wagner, N, Wagner, KD. Pharmacological Utility of PPAR Modulation for Angiogenesis in Cardiovascular Disease. Int J Mol Sci. 2023;24 (3):. doi: 10.3390/ijms24032345. PubMed PMID:36768666 PubMed Central PMC9916802.
- Safwan-Zaiter, H, Wagner, N, Wagner, KD. P16INK4A-More Than a Senescence Marker. Life (Basel). 2022;12 (9):. doi: 10.3390/life12091332. PubMed PMID:36143369 PubMed Central PMC9501954.
- Wagner, N, Wagner, KD. Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer. Cells. 2022;11 (15):. doi: 10.3390/cells11152432. PubMed PMID:35954274 PubMed Central PMC9368267.
- Wagner, KD. The Editor's Choice Articles-Section "Cells of the Cardiovascular System" 2020-2021. Cells. 2022;11 (14):. doi: 10.3390/cells11142173. PubMed PMID:35883616 PubMed Central PMC9323559.
- Wagner, KD, Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells. 2022;11 (12):. doi: 10.3390/cells11121966. PubMed PMID:35741095 PubMed Central PMC9221567.
- Wagner, N, Wagner, KD. Transcriptional Regulation of Cardiac Development and Disease. Int J Mol Sci. 2022;23 (6):. doi: 10.3390/ijms23062945. PubMed PMID:35328365 PubMed Central PMC8953235.
- Safwan-Zaiter, H, Wagner, N, Michiels, JF, Wagner, KD. Dynamic Spatiotemporal Expression Pattern of the Senescence-Associated Factor p16Ink4a in Development and Aging. Cells. 2022;11 (3):. doi: 10.3390/cells11030541. PubMed PMID:35159350 PubMed Central PMC8833900.
- Wagner, N, Wagner, KD. Every Beat You Take-The Wilms' Tumor Suppressor WT1 and the Heart. Int J Mol Sci. 2021;22 (14):. doi: 10.3390/ijms22147675. PubMed PMID:34299295 PubMed Central PMC8306835.
- Wagner, N, Ninkov, M, Vukolic, A, Cubukcuoglu Deniz, G, Rassoulzadegan, M, Michiels, JF et al.. Implications of the Wilms' Tumor Suppressor Wt1 in Cardiomyocyte Differentiation. Int J Mol Sci. 2021;22 (9):. doi: 10.3390/ijms22094346. PubMed PMID:33919406 PubMed Central PMC8122684.
- Ghanbarian, H, Aghamiri, S, Eftekhary, M, Wagner, N, Wagner, KD. Small Activating RNAs: Towards the Development of New Therapeutic Agents and Clinical Treatments. Cells. 2021;10 (3):. doi: 10.3390/cells10030591. PubMed PMID:33800164 PubMed Central PMC8001863.
- Wagner, KD, Wagner, N. PPARs and Myocardial Infarction. Int J Mol Sci. 2020;21 (24):. doi: 10.3390/ijms21249436. PubMed PMID:33322384 PubMed Central PMC7764190.
- Wagner, N, Wagner, KD. The Role of PPARs in Disease. Cells. 2020;9 (11):. doi: 10.3390/cells9112367. PubMed PMID:33126411 PubMed Central PMC7692109.
2015 - Grand Prize in Cancerology from the Simone and Cino del Duca Foundation - French Academy of Sciences
2008 - Du Bois-Reymond-Prize - German Physiological Society
2007 - AVENIR - Inserm
2003 - Long-term Fellow - EMBO
1998 - Robert-Koch-Prize, Charité, Germany

Nicole Wagner, iBV researcher, presented the promises of cancer immunotherapy – The Evening of Hope, Monaco. October, 16th 2018
Read More
iBV - Institut de Biologie Valrose
"Tour Pasteur"
Université Nice Sophia Antipolis
Faculté de médecine
28 Avenue de Valombrose
06189 Nice cedex 2
