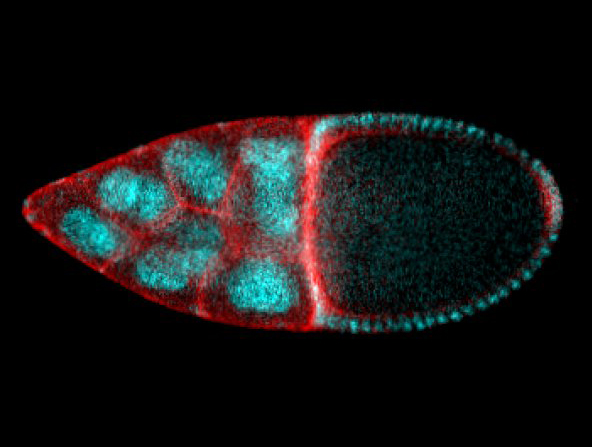
Thanks to an interdisciplinary and exciting collaboration between the iBV and the CBI in Toulouse, the Rauzi and the Wang teams unveiled a Cdc42 dependent mechanical process responsible for tissue elongation in the developing Drosophila egg chamber. By engineering new optogenetic techniques and infra-red laser based surgery, Anna Popkova and others show that a polarized supracellular actomyosin network, working as a molecular corset, generates tissue scale forces directing egg chamber elongation. Finally, this study opens new avenues to better understand how supra-cellular cytoskeletal networks emerge to drive embryo-scale morphogenesis during development. This work was published in Nature Communications.
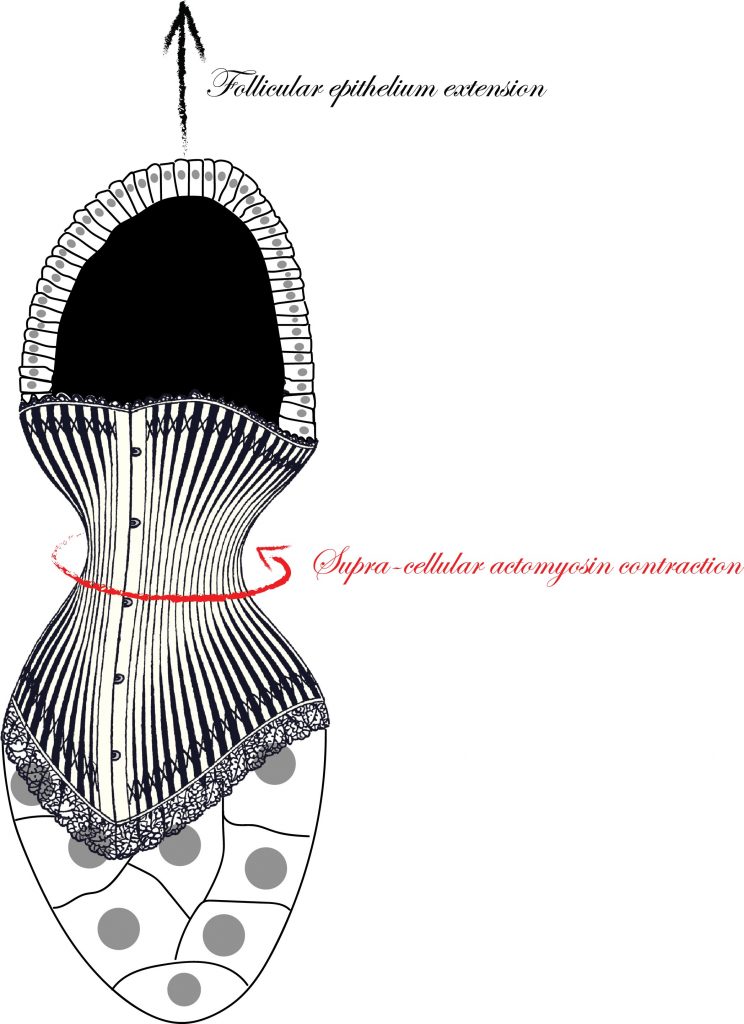
Understanding the mechanisms that are responsible to shape tissues and organs is an important and exciting challenge in developmental biology. The actomyosin cortex often plays a key role in generating the forces necessary to shape individual cells. Nevertheless, the actomyosin architecture can extend beyond the size of a single cell: cytoskeletal networks emerge at the scale of a tissue. These supra-cellular contractile structures can generate large forces that can rapidly shape epithelia.
In this study, scientists from the Rauzi and the Wang lab use the Drosophila egg chamber as a model system to study the origin and the mechanics of supra-cellular actomyosin networks. Popkova and others show that a supra-cellular array of parallel actin bundles, emerging from interdigitating filopodia, envelopes the follicle tissue. Filopodia radiate in a polarized way from basal stress fibers and extend by penetrating the neighboring cell cortexes. Filopodia can be mechanosensitive and function as anchors between cells. The small GTPase Cdc42 governs the formation of intercellular filopodia and stress fibers in the follicular cells. Thus, a Cdc42-dependent supracellular cytoskeletal network provides a scaffold integrating local oscillatory actomyosin contractions at the tissue scale to drive global polarized forces and tissue elongation.
Inset
Actin filaments assemble into diverse protrusive and contractile networks to generate forces in diverse cellular processes. Stress fibers are contractile higher order cytoskeletal structures composed of actomyosin bundles. Stress fibers play a key role in generating forces along the fiber direction and have important implication in cell adhesion to the extracellular matrix.
Filopodia are dynamic, finger-like plasma membrane protrusions that act as antennae to sense the mechanical and chemical environment. They are often regarded as “sensory organelles”. Filopodia are involved in many biological processes, such as growth cone guidance, cell migration, wound closure, and macrophage-induced cell invasion. These thin membrane protrusions are 60–200 nm in diameter and contain parallel bundles of 10–30 actin filaments held together by actin-binding proteins.
Laser dissection is a useful tool in developmental biology to probe the mechanical forces from the subcellular to the tissue/embryo scale. During tissue morphogenesis, cells are equipped with actomyosin networks generating forces. In this study, researchers used near-infrared (NIR) femtosecond (fs) pulsed laser surgery to dissect the actomyosin cytoskeleton with subcellular precision. This technique allows to selectively ablate actomyosin networks while preserving the cell plasma membrane. The resulting recoil of the remaining network, after laser dissection, is imaged and analyzed to deduce local forces responsible for tissue morphogenesis.
Optogenetics allows to control, via light stimulation, protein conformation changes and thus protein activity with spatial and temporal specificity. By using molecular engineering, proteins of interest are fused to photo-activatable proteins than can be expressed in specific cells. Using the optogenetic tool PA-Cdc42 which leads to the expression of a light-activatable Cdc42 protein, researchers were able to precisely determine the role of Cdc42 in follicular cells.
To read more:
A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension.
Popkova A, Stone OJ, Chen L, Qin X, Liu C, Liu J, Belguise K, Montell DJ, Hahn KM, Rauzi M@, Wang X@.
Nat Commun. 2020 Apr 21;11(1):1921. doi: 10.1038/s41467-020-15593-2.
Press release : Actualités scientifiques de l’INSB
Movie: Time-lapse of a representative mCD8GFP-expressing egg chamber labelled with MyoII-mCherry. Laser dissection of the supra-cellular actomyosin network was performed along the AP axis of the egg chamber. Scale bar 10 μm.