
Robert ARKOWITZ
Polarized Growth in Yeast
Main interests
- Transduction of external stimuli into asymmetric growth that is tightly controlled in time and space
- Coordination and regulation of membrane traffic resulting in distinct cell shape changes
- Temporal and spatial regulation of forces involved in invasive growth
- Interplay between force generation, cell polarity/cell organization and membrane traffic
Scientific Questions
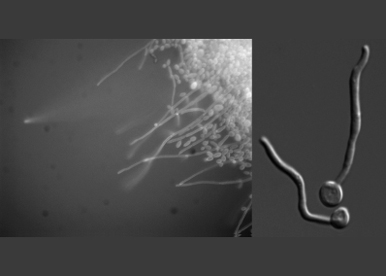
Our main interest is how cells spatially and temporally regulate growth. Polarized growth is essential for both internal organization and generation of complex multi-cellular structures. Asymmetric growth requires the specification of a polarity site, orientation of the cytoskeleton towards this site and subsequent directed membrane traffic. Our primary focus is on polarized growth, morphogenesis and development in response to external cues, predominantly in the human pathogenic yeast Candida albicans but also in the baker’s yeast Saccharomyces cerevisiae. The human commensal C. albicans switches from an oval yeast form to a hyphal filamentous form that can invade tissue and evade host immune cells. This dimorphic switch is critical for pathogenicity of C. albicans, which is a major cause of life-threatening nosocomial infections as well as persistent mucosal infections. We are investigating the roles of polarity, membrane traffic and mechanical forces in this human fungal pathogen.
Our Strategy
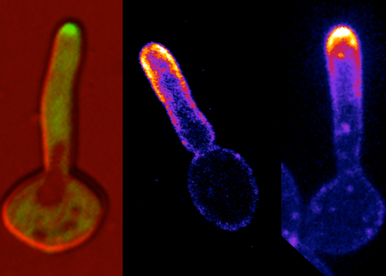
We take advantage of a range of genetic and chemical perturbations to probe cell polarity, membrane traffic and invasive growth in C. albicans. Molecular genetic approaches are used to generate mutants and fluorescent reporter fusions. A variety of microscopy techniques are used to probe the dynamics of cell polarity, membrane traffic and invasive growth with high temporal and spatial resolution. An assortment of reporters for lipid and active small GTPase distribution are optimized and used to follow establishment and maintenance of cellular asymmetries in vivo. In addition, micro-fabrication approaches are used to impose and vary physical constraints in these studies. We also work in close collaboration physicists to model aspects of cell polarity, membrane traffic and invasive growth in order to predict key parameters in these processes that will be subsequently tested.
Research Aims
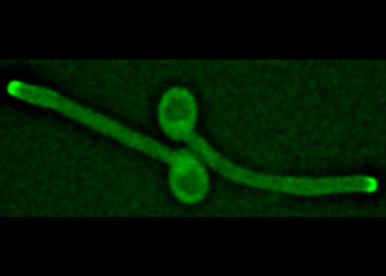
Polarity
- Role of GTPases and lipids in initiation and maintenance of asymmetric growth.
- Cellular reorganization upon cell shape changes.
- Importance of cell polarity in multicellular structures.
- Regulation of cell polarity upon external stimuli or changes in the environment, including host cells.
- Role of cell polarity in force generation and regulation of membrane traffic.
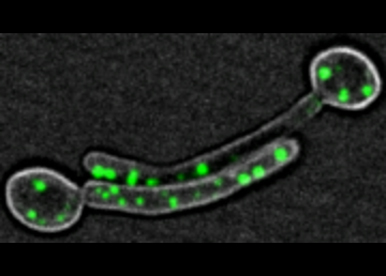
Membrane traffic
- Regulation of membrane traffic during yeast to hyphal transition.
- Membrane traffic dynamics during invasion and infection.
- Coordination of membrane traffic in multicellular structures.
- Traffic and transport of membrane lipids and their importance in hyphal growth.
- Local control of membrane traffic for site-specific growth and cell shape changes.
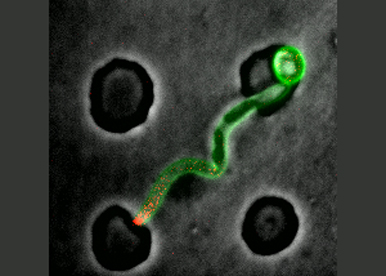
Forces
- Forces critical for invasive growth.
- Effect of resistive forces on cellular organization and polarity.
- Role of local alterations or perturbations of resistive and cellular forces during substrate penetration and invasion.
- Importance of membrane traffic and cell wall for invasive growth.
- Cellular forces and interplay with resistive forces during different types of infection.
Researchers
 BASSILANA Martine - +33 489150742
BASSILANA Martine - +33 489150742 BASSILANA Martine - +33 489150742
BASSILANA Martine - +33 489150742 BASSILANA Martine - +33 489150742
BASSILANA Martine - +33 489150742 FOLLETTE Peter - +33 489150741
FOLLETTE Peter - +33 489150741
Postdocs
 JAITLY Priya - +33 489150741
JAITLY Priya - +33 489150741 CHEVALIER Louis - +33 489150741
CHEVALIER Louis - +33 489150741
PreDocs
 PLUMB Emily - +33 489150741
PLUMB Emily - +33 489150741
Engineers & Technicians
 BOGLIOLO Stéphanie - +33 489150741
BOGLIOLO Stéphanie - +33 489150741 LABBAOUI Hayet - +33 489150741
LABBAOUI Hayet - +33 489150741 LABBAOUI Hayet - +33 489150741
LABBAOUI Hayet - +33 489150741
Recent publications
- Serrano, A, Basante-Bedoya, MA, Bassilana, M, Arkowitz, RA. A live-cell ergosterol reporter for visualization of the effects of fluconazole on the human fungal pathogen Candida albicans. mBio. 2023;14 (6):e0249323. doi: 10.1128/mbio.02493-23. PubMed PMID:38032182 PubMed Central PMC10746211.
- Alder-Rangel, A, Bailão, AM, Herrera-Estrella, A, Rangel, AEA, Gácser, A, Gasch, AP et al.. The IV International Symposium on Fungal Stress and the XIII International Fungal Biology Conference. Fungal Biol. 2023;127 (7-8):1157-1179. doi: 10.1016/j.funbio.2023.04.006. PubMed PMID:37495306 PubMed Central PMC11668258.
- Basante-Bedoya, MA, Bogliolo, S, Garcia-Rodas, R, Zaragoza, O, Arkowitz, RA, Bassilana, M et al.. Two distinct lipid transporters together regulate invasive filamentous growth in the human fungal pathogen Candida albicans. PLoS Genet. 2022;18 (12):e1010549. doi: 10.1371/journal.pgen.1010549. PubMed PMID:36516161 PubMed Central PMC9797089.
- Garcia-Rodas, R, Labbaoui, H, Orange, F, Solis, N, Zaragoza, O, Filler, SG et al.. Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection. mBio. 2021;13 (1):e0387321. doi: 10.1128/mbio.03873-21. PubMed PMID:35164565 PubMed Central PMC8942462.
- Puerner, C, Serrano, A, Wakade, RS, Bassilana, M, Arkowitz, RA. A Myosin Light Chain Is Critical for Fungal Growth Robustness in Candida albicans. mBio. 2021;12 (5):e0252821. doi: 10.1128/mBio.02528-21. PubMed PMID:34607458 PubMed Central PMC8546852.
- Abdul-Ganiyu, R, Venegas, LA, Wang, X, Puerner, C, Arkowitz, RA, Kay, BK et al.. Phosphorylated Gβ is a directional cue during yeast gradient tracking. Sci Signal. 2021;14 (682):. doi: 10.1126/scisignal.abf4710. PubMed PMID:33975981 .
- Puerner, C, Kukhaleishvili, N, Thomson, D, Schaub, S, Noblin, X, Seminara, A et al.. Mechanical force-induced morphology changes in a human fungal pathogen. BMC Biol. 2020;18 (1):122. doi: 10.1186/s12915-020-00833-0. PubMed PMID:32912212 PubMed Central PMC7488538.
- Etienne-Manneville, S, Arkowitz, R. Cell polarity inside-out. Curr Opin Cell Biol. 2020;62 :iii-iv. doi: 10.1016/j.ceb.2020.01.008. PubMed PMID:32081296 .
- Bassilana, M, Puerner, C, Arkowitz, RA. External signal-mediated polarized growth in fungi. Curr Opin Cell Biol. 2020;62 :150-158. doi: 10.1016/j.ceb.2019.11.001. PubMed PMID:31875532 .
- Silva, PM, Puerner, C, Seminara, A, Bassilana, M, Arkowitz, RA. Secretory Vesicle Clustering in Fungal Filamentous Cells Does Not Require Directional Growth. Cell Rep. 2019;28 (8):2231-2245.e5. doi: 10.1016/j.celrep.2019.07.062. PubMed PMID:31433995 .
- Arkowitz, RA, Bassilana, M. Recent advances in understanding Candida albicans hyphal growth. F1000Res. 2019;8 :. doi: 10.12688/f1000research.18546.1. PubMed PMID:31131089 PubMed Central PMC6530606.
- Weiner, A, Orange, F, Lacas-Gervais, S, Rechav, K, Ghugtyal, V, Bassilana, M et al.. On-site secretory vesicle delivery drives filamentous growth in the fungal pathogen Candida albicans. Cell Microbiol. 2019;21 (1):e12963. doi: 10.1111/cmi.12963. PubMed PMID:30321912 .
- Bar-Yosef, H, Gildor, T, Ramírez-Zavala, B, Schmauch, C, Weissman, Z, Pinsky, M et al.. A Global Analysis of Kinase Function in Candida albicans Hyphal Morphogenesis Reveals a Role for the Endocytosis Regulator Akl1. Front Cell Infect Microbiol. 2018;8 :17. doi: 10.3389/fcimb.2018.00017. PubMed PMID:29473018 PubMed Central PMC5809406.
- Wakade, R, Labbaoui, H, Stalder, D, Arkowitz, RA, Bassilana, M. Overexpression of YPT6 restores invasive filamentous growth and secretory vesicle clustering in a Candida albicans arl1 mutant. Small GTPases. 2020;11 (3):204-210. doi: 10.1080/21541248.2017.1378157. PubMed PMID:28960163 PubMed Central PMC7549723.
- Labbaoui, H, Bogliolo, S, Ghugtyal, V, Solis, NV, Filler, SG, Arkowitz, RA et al.. Role of Arf GTPases in fungal morphogenesis and virulence. PLoS Pathog. 2017;13 (2):e1006205. doi: 10.1371/journal.ppat.1006205. PubMed PMID:28192532 PubMed Central PMC5325608.
- Lin, TC, Neuner, A, Flemming, D, Liu, P, Chinen, T, Jäkle, U et al.. MOZART1 and γ-tubulin complex receptors are both required to turn γ-TuSC into an active microtubule nucleation template. J Cell Biol. 2016;215 (6):823-840. doi: 10.1083/jcb.201606092. PubMed PMID:27920216 PubMed Central PMC5166503.
- Stone, DE, Arkowitz, RA. In Situ Assays of Chemotropism During Yeast Mating. Methods Mol Biol. 2016;1407 :1-12. doi: 10.1007/978-1-4939-3480-5_1. PubMed PMID:27271890 .
- Ismael, A, Tian, W, Waszczak, N, Wang, X, Cao, Y, Suchkov, D et al.. Gβ promotes pheromone receptor polarization and yeast chemotropism by inhibiting receptor phosphorylation. Sci Signal. 2016;9 (423):ra38. doi: 10.1126/scisignal.aad4376. PubMed PMID:27072657 PubMed Central PMC4908976.
- Ghugtyal, V, Garcia-Rodas, R, Seminara, A, Schaub, S, Bassilana, M, Arkowitz, RA et al.. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc Natl Acad Sci U S A. 2015;112 (28):8644-9. doi: 10.1073/pnas.1504259112. PubMed PMID:26124136 PubMed Central PMC4507248.
- Arkowitz, RA, Bassilana, M. Rho GTPase-phosphatidylinositol phosphate interplay in fungal cell polarity. Biochem Soc Trans. 2014;42 (1):206-11. doi: 10.1042/BST20130226. PubMed PMID:24450653 .
2024 - Emily Plumb, Doctorante: prix du meilleur poster symposium "Microbes 2024", Association Francophone d'Ecologie Microbienne
2022 - GSA Poster Award winners - Antonio Serrano-Salces - 31st Fungal Genetics Conference
2021 - Elected fellow of the American Academy of Microbiology (AAM)
2015 - Marie Curie Multi-ITN (H2020-MSCA-ITN-2015)
2013 - Marie Curie Multi-ITN (FP7-PEOPLE-2013-ITN)
2012 - France Berkeley Fund Award
2005 - Marie Curie Host Fellowships for Early Stage Researchers (EST). FP6-2002-Mobility 2
2002 - Equipe Labellisée - La Ligue Contre le Cancer
2001 - Young Investigator Programme - EMBO
2001 - France Berkeley Fund Award
2001 - FRM Fondation pour la Recherche Médicale – BNP Paribas Award
2000 - Installation Award - FRM
2000 - ATIP - CNRS
1998 - EU Eurofan II (European Network for the Functional Analysis of Yeast Genes) FP5

Antonio SERRANO SALCES: Understanding morphogenesis in the human pathogenic fungus Candida albicans
Read More

Rob Arkowitz elected fellow of the American Academy of Microbiology (AAM)
Read More
Resetting cell polarity with light
Read More
iBV - Institut de Biologie Valrose
"Centre de Biochimie"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
