
Andreas SCHEDL
Molecular programs controlling development and tissue homeostasis
Main interests
- Signals directing self-renewal and differentiation of renal stem cells
- Transcriptional control of glomerular podocyte differentiation
- Adrenal tissue homeostasis and its links to cancer
- Sex-specific differences of non-reproductive organs
Scientific Questions
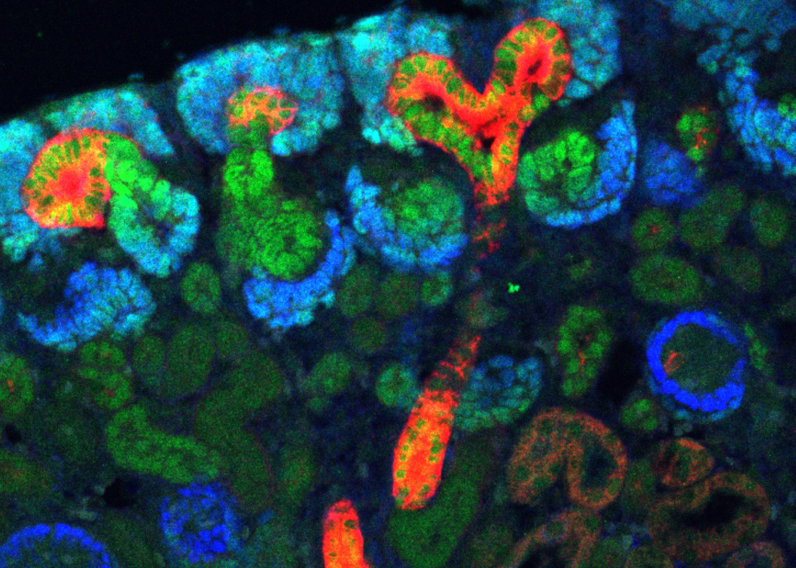
Organ development is a highly complex process that requires the orchestrated action of transcription factors and signalling pathways. Once an organ has formed it needs to be maintained to ensure proper functioning throughout the entire life. To achieve this feat, many organs employ stem cells that can be activated to renew the damaged organ. Past research has demonstrated that development and tissue maintenance are highly interrelated and molecular programs driving development and differentiation are also triggered when stem cells become activated to replace damaged or lost cells. Focusing on the kidney and adrenal glands, our research program aims at understanding the transcriptional control underlying tissue development, define stem/progenitor cells in the adult organism and determine the signalling pathways that are involved in their maintenance and activation.
Our Strategy
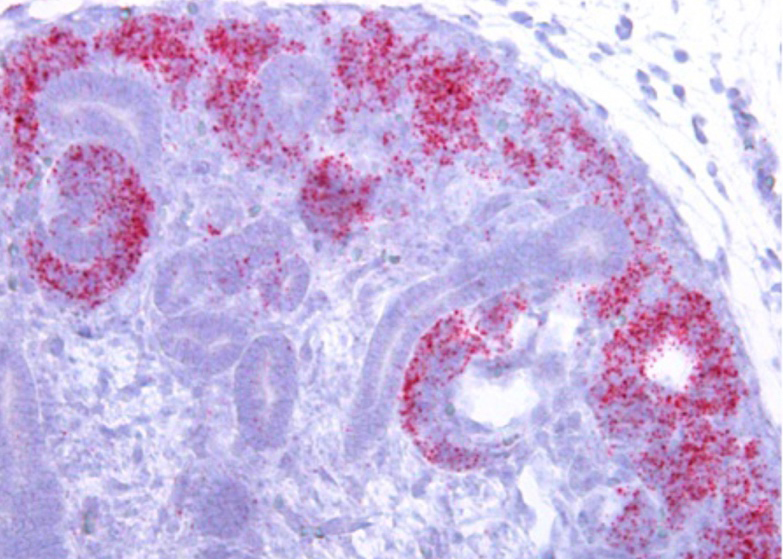
Kidneys are central cardiovascular organs that ensure blood filtration and - together with the adrenals - control blood pressure, pH and electrolyte concentration. Kidney diseases are on the rise and it is expected that as many as 1 in 10 people will suffer from renal disease at some stage during their life. Moreover, defective blood pressure control leads to hypertension, the foremost cause of death worldwide. Given the high incidence of these diseases, new therapeutic approaches are urgently required. A vision for the future is to employ regenerative medicine that would permit the growth of new organs from a patient’s own cells. To achieve this goal an in depth knowledge of how kidneys and adrenals form and how they are maintainted is required. In our lab, we are concentrating on three main topics: 1) We address how proliferation of renal stem cells is regulated and identify molecular networks directing their differentiation into functional nephrons. 2) We study podocytes, a key component of the renal filtration barrier, and address how differentiation and maintenance of this unique cell type is achieved. 3) We identify the molecular pathways required for adrenal cortex specification and maintenance. To achieve our goals we employ state of the art genetic (inducible knockouts, CRISPR/Cas9, lineage tracing, organoids) and molecular techniques (ChIP-Seq, scRNA-Seq) and analyze gene function in vitro and in vivo.
Research Aims
Nephron progenitors represent a developmental stem cell population that fuels nephron formation and determines the total number of nephrons per kidney. Progenitors need to constantly decide between quiescence, proliferation and differentiation, which is achieved through signalling pathways that induce specific transcriptional responses. We are focussing on the transcriptional regulators of the SOXC class, identify their role in these processes and determine the molecular networks they regulate.
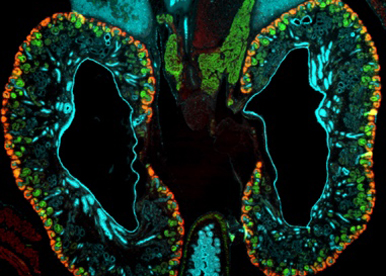
Podocytes are highly specialized cells that form unique cell extensions (footprocesses) required for glomerular filtration. Podocyte damage is one of the main mechanisms leading to end stage renal failure thus underlining the importance of this cell type. We are interested in identifying the transcriptional program orchestrating podocyte differentiation and maintenance and understand how the unique structure and function of this cell type is acquired and maintained.
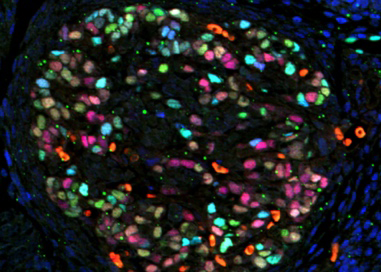
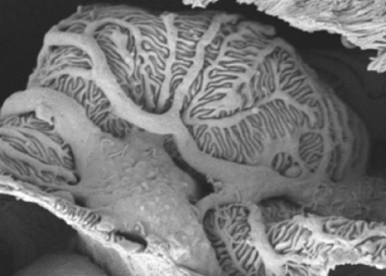
The adult adrenal cortex undergoes constant tissue renewal. To achieve this feat cells proliferate at the outer cortex and migrate in a centripetal fashion changing their differentiation along their differentiation path only to undergo apoptosis at the cortico-medullary boundary. We are studying the molecular pathways that ensure maintenance of organ size and function, and address how disruption of tissue dynamics can lead to adrenal diseases, including cancer.
Researchers
 VIDAL Valérie - +33 489150735
VIDAL Valérie - +33 489150735 NEIRIJNCK Yasmine - +33 489150735
NEIRIJNCK Yasmine - +33 489150735
PreDocs
 TEDESCO Mélina - +33 R
TEDESCO Mélina - +33 R KOVáCS Kristóf - +33 R
KOVáCS Kristóf - +33 R
Engineers & Technicians
 JIAN MOTAMEDI Fariba - +33 489150735
JIAN MOTAMEDI Fariba - +33 489150735 BENOIT Timothé - +33 489150873
BENOIT Timothé - +33 489150873 BENOIT Timothé - +33 489150873
BENOIT Timothé - +33 489150873 JAILLOT Léa - +33 R
JAILLOT Léa - +33 R
Recent Publications
- Ediga, HH, Vemulapalli, CP, Sontake, V, Patel, PK, Miyazaki, H, Popov, D et al.. Wilms' tumor 1 impairs apoptotic clearance of fibroblasts in distal fibrotic lung lesions. J Clin Invest. 2025; :. doi: 10.1172/JCI188819. PubMed PMID:40493404 .
- Oikonomakos, I, Tedesco, M, Motamedi, FJ, Peitzsch, M, Nef, S, Bornstein, SR et al.. In vitro differentiation of mouse pluripotent stem cells into corticosteroid-producing adrenocortical cells. Stem Cell Reports. 2024;19 (9):1289-1303. doi: 10.1016/j.stemcr.2024.07.010. PubMed PMID:39178848 PubMed Central PMC11411339.
- Gregoire, EP, De Cian, MC, Migale, R, Perea-Gomez, A, Schaub, S, Bellido-Carreras, N et al.. The -KTS splice variant of WT1 is essential for ovarian determination in mice. Science. 2023;382 (6670):600-606. doi: 10.1126/science.add8831. PubMed PMID:37917714 PubMed Central PMC7615308.
- Bechmann, N, Moskopp, ML, Constantinescu, G, Stell, A, Ernst, A, Berthold, F et al.. Asymmetric Adrenals: Sexual Dimorphism of Adrenal Tumors. J Clin Endocrinol Metab. 2024;109 (2):471-482. doi: 10.1210/clinem/dgad515. PubMed PMID:37647861 PubMed Central PMC11032253.
- Lyraki, R, Grabek, A, Tison, A, Weerasinghe Arachchige, LC, Peitzsch, M, Bechmann, N et al.. Crosstalk between androgen receptor and WNT/β-catenin signaling causes sex-specific adrenocortical hyperplasia in mice. Dis Model Mech. 2023;16 (6):. doi: 10.1242/dmm.050053. PubMed PMID:37102205 PubMed Central PMC10184674.
- Neirijnck, Y, Sararols, P, Kühne, F, Mayère, C, Weerasinghe Arachchige, LC, Regard, V et al.. Single-cell transcriptomic profiling redefines the origin and specification of early adrenogonadal progenitors. Cell Rep. 2023;42 (3):112191. doi: 10.1016/j.celrep.2023.112191. PubMed PMID:36862551 .
- Neirijnck, Y, Schedl, A. Rats, Adrenals, and the Surprising Role of the Corticosteroid-Binding Globulin in Sexual Dimorphism. Endocrinology. 2022;164 (1):. doi: 10.1210/endocr/bqac190. PubMed PMID:36402142 .
- Bechmann, N, Barthel, A, Schedl, A, Herzig, S, Varga, Z, Gebhard, C et al.. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022;10 (3):221-230. doi: 10.1016/S2213-8587(21)00346-6. PubMed PMID:35114136 PubMed Central PMC8803381.
- Martinez, A, Schedl, A. Dissecting a zonated organ - Special issue on adrenal biology. Mol Cell Endocrinol. 2022;539 :111486. doi: 10.1016/j.mce.2021.111486. PubMed PMID:34626732 .
- Da Silva, F, Jian Motamedi, F, Weerasinghe Arachchige, LC, Tison, A, Bradford, ST, Lefebvre, J et al.. Retinoic acid signaling is directly activated in cardiomyocytes and protects mouse hearts from apoptosis after myocardial infarction. Elife. 2021;10 :. doi: 10.7554/eLife.68280. PubMed PMID:34623260 PubMed Central PMC8530512.
- Da Silva, F, Zhang, K, Pinson, A, Fatti, E, Wilsch-Bräuninger, M, Herbst, J et al.. Mitotic WNT signalling orchestrates neurogenesis in the developing neocortex. EMBO J. 2021;40 (19):e108041. doi: 10.15252/embj.2021108041. PubMed PMID:34431536 PubMed Central PMC8488556.
- Le Rolle, M, Massa, F, Siggers, P, Turchi, L, Loubat, A, Koo, BK et al.. Arrest of WNT/β-catenin signaling enables the transition from pluripotent to differentiated germ cells in mouse ovaries. Proc Natl Acad Sci U S A. 2021;118 (30):. doi: 10.1073/pnas.2023376118. PubMed PMID:34301885 PubMed Central PMC8325354.
- Lyraki, R, Schedl, A. The Sexually Dimorphic Adrenal Cortex: Implications for Adrenal Disease. Int J Mol Sci. 2021;22 (9):. doi: 10.3390/ijms22094889. PubMed PMID:34063067 PubMed Central PMC8124132.
- Lyraki, R, Schedl, A. Adrenal cortex renewal in health and disease. Nat Rev Endocrinol. 2021;17 (7):421-434. doi: 10.1038/s41574-021-00491-4. PubMed PMID:34011989 .
- Oikonomakos, I, Weerasinghe Arachchige, LC, Schedl, A. Developmental mechanisms of adrenal cortex formation and their links with adult progenitor populations. Mol Cell Endocrinol. 2021;524 :111172. doi: 10.1016/j.mce.2021.111172. PubMed PMID:33484742 .
- Russell, JP, Lim, X, Santambrogio, A, Yianni, V, Kemkem, Y, Wang, B et al.. Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells. Elife. 2021;10 :. doi: 10.7554/eLife.59142. PubMed PMID:33399538 PubMed Central PMC7803373.
- Comai, GE, Tesařová, M, Dupé, V, Rhinn, M, Vallecillo-García, P, da Silva, F et al.. Local retinoic acid signaling directs emergence of the extraocular muscle functional unit. PLoS Biol. 2020;18 (11):e3000902. doi: 10.1371/journal.pbio.3000902. PubMed PMID:33201874 PubMed Central PMC7707851.
- Werdermann, M, Berger, I, Scriba, LD, Santambrogio, A, Schlinkert, P, Brendel, H et al.. Insulin and obesity transform hypothalamic-pituitary-adrenal axis stemness and function in a hyperactive state. Mol Metab. 2021;43 :101112. doi: 10.1016/j.molmet.2020.101112. PubMed PMID:33157254 PubMed Central PMC7691554.
- Bornstein, SR, Malyukov, M, Heller, C, Ziegler, CG, Ruiz-Babot, G, Schedl, A et al.. New Horizons: Novel Adrenal Regenerative Therapies. J Clin Endocrinol Metab. 2020;105 (9):3103-7. doi: 10.1210/clinem/dgaa438. PubMed PMID:32629476 PubMed Central PMC7398608.
- Chassot, AA, Le Rolle, M, Jolivet, G, Stevant, I, Guigonis, JM, Da Silva, F et al.. Retinoic acid synthesis by ALDH1A proteins is dispensable for meiosis initiation in the mouse fetal ovary. Sci Adv. 2020;6 (21):eaaz1261. doi: 10.1126/sciadv.aaz1261. PubMed PMID:32494737 PubMed Central PMC7244317.
2018 - Equipe labellisée – Ligue contre le Cancer
2012 - Equipe labellisée - ARC
2009 - Award of the French kidney foundation (Prix de la Fondation du Rein)
2009 - Equipe labellisée - FRM
2007 - Member of the Faculty of 1000 Medecine
2006 - Equipe labellisée - FRM
2003 - Avenir - Inserm
2002 - Philippe Leverhulme Prize (UK)
2001 - Young Investigator Programme - EMBO

Congratulations to Yasmine Neirijnck and Agnes Banreti for their INSERM permanent positions!
Read More
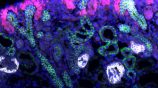
Novel factors discovered that orchestrate kidney formation
Read More
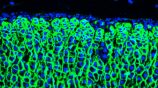
Why are women more at risk of adrenal cancer?
Read More

UCA annual Award Ceremony: 7 iBV members recognised for their scientific contributions
Read More
iBV - Institut de Biologie Valrose
"Centre de Biochimie"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
