
Patrick COLLOMBAT
Diabetes Genetics
Main interests
- Diabetes
- Regeneration
- Pancreas
- Mouse development
Scientific Questions
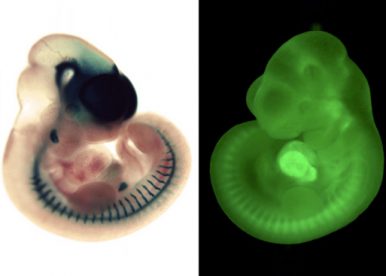
The pancreas can be subdivided into two distinct structures: the exocrine and the endocrine tissues. The exocrine compartment secretes and transports digestive enzymes to the duodenum, whereas the endocrine tissue is organized into small clusters of cells termed islets of Langerhans. The latter contain four main different hormone-secreting cell subtypes: alpha-, beta-, delta-, and PP-cells producing glucagon, insulin, somatostatin, and pancreatic polypeptide (PP), respectively.
Both type I and type II diabetes conditions may ultimately result in pancreatic beta-cell loss and chronic hyperglycemia. While current therapies (insulin supplementation, transplantation) provide a measure of control of the glycemia, treated diabetic patients still display a shortened life expectancy and worsened quality of life as compared to their healthy counterparts.
Our research therefore aims at finding alternative ways to treat type 1 diabetes through the induction of beta-cell regeneration.
Our Strategy
Using the mouse as a model, we have initially focused our research on the Arx and Pax4 transcription factors. Importantly, we demonstrated that :
- both factors control endocrine cell development during pancreas morphogenesis, Arx inducing the alpha-cell lineage, whereas Pax4 promotes the beta- and delta-cell fates.
- the forced expression of the Arx gene in adult beta-cells convert these into cells displaying an alpha- or PP-cell phenotype in vivo.
- the misexpression of Pax4 gene in embryonic (Collombat et al., Cell, 2009) or adult alpha-cells induces their continued regeneration and conversion into beta-like cells, such cells being able to reverse several rounds of chemically-induced diabetes in vivo.
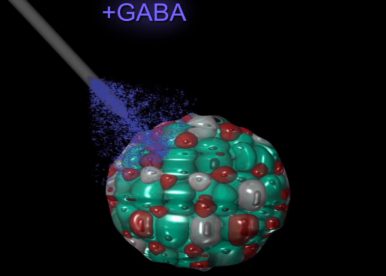
Importantly, through a collaborative screening effort, we recently identified several chemical compounds of interest for diabetes research (Ben-Othman et al, Cell, 2017 - Li et al, Cell, 2017). Among these, GABA (γ-aminobutyric acid - a well know neurotransmitter also produced in the pancreas) was found able to induce alpha-cell-mediated beta-like cell neogenesis. Indeed, when provided to animals rendered diabetics, GABA could literally induce the regeneration of a functional beta-cell mass and thereby reverse the consequences of diabetes (multiple times) in vivo.
We are currently deciphering the genetic cascade underlying GABA signalling and running a pilot clinical trial aiming to determine whether GABA could potentially help diabetic patients.
Research Aims
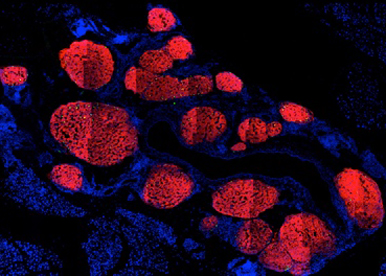
On main focus is to establish ways to induce pancreatic beta-cell regeneration in the context of diabetes research. We have obtained very promising results with the discovery of GABA but we have addional candidates to test. In addition, we work hard in order to understand how GABA works and whether its activities could be potentiated and/or improved.
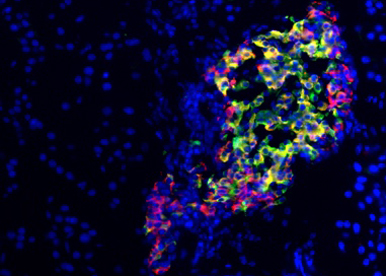
Using different mouse models combined to human islet studies, we are caracterising the consequences of genetic manipulations of a number of genes on type 1 and type 2 diabetes onset. We have several gene candidates that, when manipulated, could be benificial for both types of diabetes.
Type 1 diabetes is an autoimmune disease targeting insulin-producing beta-cells. Finding ways to regenerate beta-cells is of crucial interest. However, we also need to consider their putative subsequent ablation by the immune system. We are therefore developing approaches allowing their protection from autoimmune attack.
Researchers
 NAPOLITANO Tiziana - +33 489150716
NAPOLITANO Tiziana - +33 489150716 SILVANO Serena - +33 489150716
SILVANO Serena - +33 489150716 AYACHI Chaïma - +33 489150716
AYACHI Chaïma - +33 489150716 TREINS Caroline - +33 489150717
TREINS Caroline - +33 489150717
Engineers & Technicians
 VERISSIMO FOFO Hugo Miguel - +33 489150716
VERISSIMO FOFO Hugo Miguel - +33 489150716 ETASSE Laura - +33 489150716
ETASSE Laura - +33 489150716 MONGIS Aline - +33 R
MONGIS Aline - +33 R MONGIS Aline - +33 R
MONGIS Aline - +33 R
Recent publications
- Silvano, S, Napolitano, T, Plaisant, M, Sousa-De-Veiga, A, Fofo, H, Ayachi, C et al.. RSPO1, a potent inducer of pancreatic β cell neogenesis. Cell Rep Med. 2025;6 (5):102126. doi: 10.1016/j.xcrm.2025.102126. PubMed PMID:40339569 PubMed Central PMC12147903.
- Oger, F, Bourouh, C, Friano, ME, Courty, E, Rolland, L, Gromada, X et al.. β-Cell-Specific E2f1 Deficiency Impairs Glucose Homeostasis, β-Cell Identity, and Insulin Secretion. Diabetes. 2023;72 (8):1112-1126. doi: 10.2337/db22-0604. PubMed PMID:37216637 .
- Napolitano, T, Silvano, S, Ayachi, C, Plaisant, M, Sousa-Da-Veiga, A, Fofo, H et al.. Wnt Pathway in Pancreatic Development and Pathophysiology. Cells. 2023;12 (4):. doi: 10.3390/cells12040565. PubMed PMID:36831232 PubMed Central PMC9954665.
- Garrido-Utrilla, A, Ayachi, C, Friano, ME, Atlija, J, Balaji, S, Napolitano, T et al.. Conversion of Gastrointestinal Somatostatin-Expressing D Cells Into Insulin-Producing Beta-Like Cells Upon Pax4 Misexpression. Front Endocrinol (Lausanne). 2022;13 :861922. doi: 10.3389/fendo.2022.861922. PubMed PMID:35573999 PubMed Central PMC9103212.
- Napolitano, T, Avolio, F, Silvano, S, Forcisi, S, Pfeifer, A, Vieira, A et al.. Gfi1 Loss Protects against Two Models of Induced Diabetes. Cells. 2021;10 (11):. doi: 10.3390/cells10112805. PubMed PMID:34831029 PubMed Central PMC8616283.
- Khurana, I, Al-Hasani, K, Maxwell, S, K N, H, Okabe, J, Cooper, ME et al.. DNA methylation status correlates with adult β-cell regeneration capacity. NPJ Regen Med. 2021;6 (1):7. doi: 10.1038/s41536-021-00119-1. PubMed PMID:33580013 PubMed Central PMC7881134.
- Marquina-Sanchez, B, Fortelny, N, Farlik, M, Vieira, A, Collombat, P, Bock, C et al.. Single-cell RNA-seq with spike-in cells enables accurate quantification of cell-specific drug effects in pancreatic islets. Genome Biol. 2020;21 (1):106. doi: 10.1186/s13059-020-02006-2. PubMed PMID:32375897 PubMed Central PMC7201533.
- Poeta, L, Padula, A, Attianese, B, Valentino, M, Verrillo, L, Filosa, S et al.. Histone demethylase KDM5C is a SAHA-sensitive central hub at the crossroads of transcriptional axes involved in multiple neurodevelopmental disorders. Hum Mol Genet. 2019;28 (24):4089-4102. doi: 10.1093/hmg/ddz254. PubMed PMID:31691806 PubMed Central PMC7002875.
- Napolitano, T, Silvano, S, Vieira, A, Balaji, S, Garrido-Utrilla, A, Friano, ME et al.. Role of ghrelin in pancreatic development and function. Diabetes Obes Metab. 2018;20 Suppl 2 :3-10. doi: 10.1111/dom.13385. PubMed PMID:30230184 .
- Balaji, S, Napolitano, T, Silvano, S, Friano, ME, Garrido-Utrilla, A, Atlija, J et al.. Epigenetic Control of Pancreatic Regeneration in Diabetes. Genes (Basel). 2018;9 (9):. doi: 10.3390/genes9090448. PubMed PMID:30205460 PubMed Central PMC6162679.
- Vieira, A, Vergoni, B, Courtney, M, Druelle, N, Gjernes, E, Hadzic, B et al.. Neurog3 misexpression unravels mouse pancreatic ductal cell plasticity. PLoS One. 2018;13 (8):e0201536. doi: 10.1371/journal.pone.0201536. PubMed PMID:30092080 PubMed Central PMC6084906.
- Druelle, N, Vieira, A, Shabro, A, Courtney, M, Mondin, M, Rekima, S et al.. Ectopic expression of Pax4 in pancreatic δ cells results in β-like cell neogenesis. J Cell Biol. 2017;216 (12):4299-4311. doi: 10.1083/jcb.201704044. PubMed PMID:29025873 PubMed Central PMC5716283.
- Vieira, A, Ben-Othman, N, Druelle, N, Courtney, M, Avolio, F, Napolitano, T et al.. [Induction of pancreatic β-like cell regeneration by activation of GABA signaling pathways]. Med Sci (Paris). 2017;33 (6-7):565-567. doi: 10.1051/medsci/20173306002. PubMed PMID:28990547 .
- Napolitano, T, Avolio, F, Vieira, A, Ben-Othman, N, Courtney, M, Gjernes, E et al.. GABA signaling stimulates α-cell-mediated β-like cell neogenesis. Commun Integr Biol. 2017;10 (3):e1300215. doi: 10.1080/19420889.2017.1300215. PubMed PMID:28702122 PubMed Central PMC5501192.
- Vieira, A, Druelle, N, Avolio, F, Napolitano, T, Navarro-Sanz, S, Silvano, S et al.. β-Cell Replacement Strategies: The Increasing Need for a "β-Cell Dogma". Front Genet. 2017;8 :75. doi: 10.3389/fgene.2017.00075. PubMed PMID:28634486 PubMed Central PMC5459879.
- Vieira, A, Ben-Othman, N, Collombat, P. GABA triggers pancreatic β-like cell neogenesis. Cell Cycle. 2017;16 (8):727-728. doi: 10.1080/15384101.2017.1302212. PubMed PMID:28272980 PubMed Central PMC5405719.
- Li, J, Casteels, T, Frogne, T, Ingvorsen, C, Honoré, C, Courtney, M et al.. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Cell. 2017;168 (1-2):86-100.e15. doi: 10.1016/j.cell.2016.11.010. PubMed PMID:27916275 PubMed Central PMC5236063.
- Ben-Othman, N, Vieira, A, Courtney, M, Record, F, Gjernes, E, Avolio, F et al.. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell. 2017;168 (1-2):73-85.e11. doi: 10.1016/j.cell.2016.11.002. PubMed PMID:27916274 .
- Ahmad, Z, Rafeeq, M, Collombat, P, Mansouri, A. Pax6 Inactivation in the Adult Pancreas Reveals Ghrelin as Endocrine Cell Maturation Marker. PLoS One. 2015;10 (12):e0144597. doi: 10.1371/journal.pone.0144597. PubMed PMID:26658466 PubMed Central PMC4676685.
- Napolitano, T, Avolio, F, Courtney, M, Vieira, A, Druelle, N, Ben-Othman, N et al.. Pax4 acts as a key player in pancreas development and plasticity. Semin Cell Dev Biol. 2015;44 :107-14. doi: 10.1016/j.semcdb.2015.08.013. PubMed PMID:26319183 .
2024 - Prix des inventeurs 2024, magazine Le Point
2015 - Prix Auguste Loubatière, Société Francophone du Diabète
2014 - Prix G. B. Morgagni
2013 - Apollinaire Bouchardat Award
2013 - Grand Prix de la Fondation Générale de Santé, Académie des Sciences
2011 - ERC Starting Grant
2009 - Schlumberger Prize
2009 - Avenir Excellency, Inserm
2009 - Career Development Award, JDRF
iBV - Institut de Biologie Valrose
"Centre de Biochimie"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
