
Guillaume SANDOZ
Biology of ion channels
Main interests
- Regulation of neuroexcitability
- Structure-function studies of ion channels
- Subunit organization of ion channels
- Channelopathy
Scientific Questions
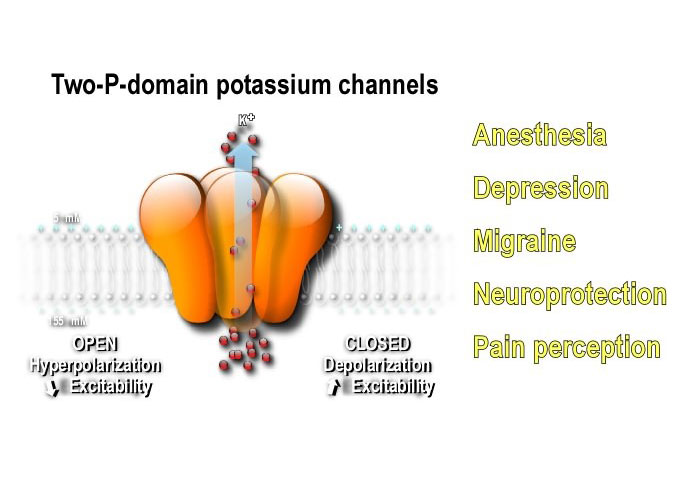
Ion channels generate the electrical signals with which the nervous system senses the world, processes information, creates memories and controls behavior. Dysfunctional ion channels can cause diseases known as channelopathies. They are encoded by approximately 350 genes and represent the second largest drug targets to notably treat diabetes, hypertension, depression, anxiety, neuropathic pain and migraine.
Our recent discoveries in the understanding of their function and regulation are opening new perspectives to treat neurological and psychiatric diseases. In fine, the challenge of the team is to integrate the structural, pharmacological, cellular and neurophysiological data of ion channel biology to promote the discovery of molecules with high therapeutic potential that are more slective and effective, reducing side effects.
Our Strategy
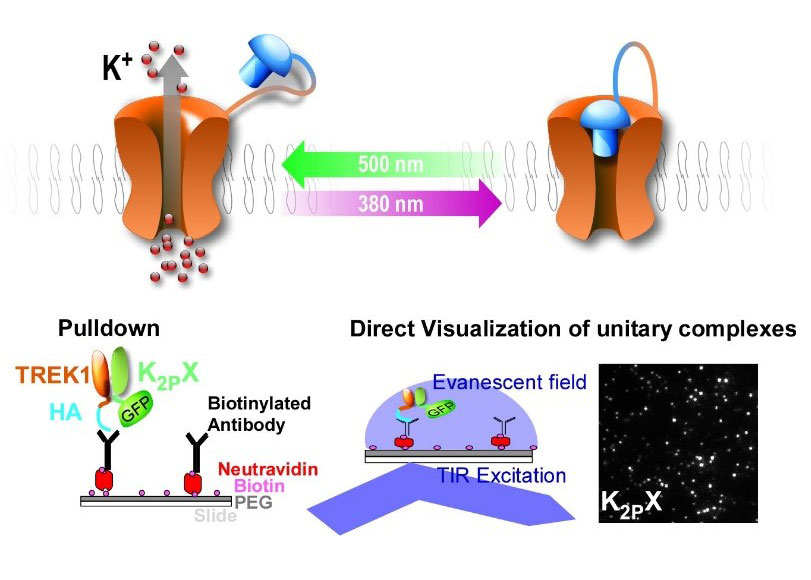
Traditional methods for probing ion channel and receptor functions in their native context require pharmacological agents or gene invalidation. However, these methods have their limitations. Many signaling proteins, including K2P channels, do not have selective ligands. Even if selective ligand exists it is hard to obtain a spatiotemporal determined effect. Furthermore, interpreting function from gene invalidation can be restricted, due to overlapping genetic function, genetic compensation and developmental issues. To overcome these limits, we developed 2 strategies.
The first one is TREK1 K2P channel that is controlled by light via a tethered photoisomerizable pore-blocker called MAQ (Figure 6). MAQ contains a maleimide (M) that tethers the molecule on the channel, a photoisomerizable azobenzene (A) linker and a pore-blocking quaternary ammonium group (Q) (Sandoz et al., 2012). This photoswitchable channel, called TREKlight, behaves like a wild-type channel in visible light or in the dark but a pulse of UV light reversibly closes it (Figure 6). This tool is used in vitro and in vivo allowing to address TREK channel role by controlling the channel function with light in a spatiotemporally defined way without any further manipulation.
The second one is to use photochromatic ligand that permit the specific light control of the activity of the K2Ps, without any genetic manipulation in mammals as well in the fish and worm.
Research Aims
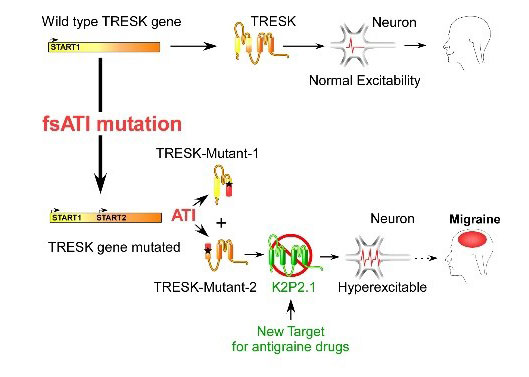
We recently demonstrated that migraine-associated frameshift mutations of TRESK, a two-pore-domain K+ channel, lead to the production of tow second protein fragments instead of one.The second fragment carries the pathophysiological function by inhibiting TREK1 and 2, and its production is due to a mechanism that we called frameshift mutation-induced Alternative Translation Initiation (fsATI). We aim now to determine if TREK agonists can be used to treat migraine.
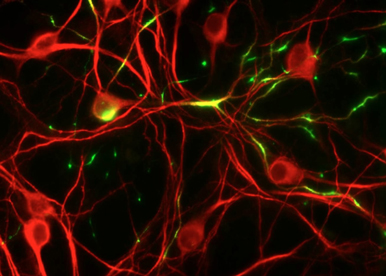
To adress K2P channel functions such as excitability, adaptation, action potential duration, synaptic transmission and plasticity, we are using fresh brain slices in which we remote control the channel activity with light.
Several members of the TREK channel subfamily, and more generally the K2P channel family, show overlapping expression patterns in many brain regions which raises the question: do these channels heteromerize and what are the functions of these heteromers? We are determining potential K2P heteromer formation and function using a combination of SiMPull and PCS
Researchers
 LANDRA WILLM Arnaud - +33 489150815
LANDRA WILLM Arnaud - +33 489150815
Engineers & Technicians
 WDZIEKONSKI Brigitte - +33 489150815
WDZIEKONSKI Brigitte - +33 489150815 CAUMONT Aurore - +33 R
CAUMONT Aurore - +33 R THOUVENIN Catherine - +33 R
THOUVENIN Catherine - +33 R
- Bied, M, Landra-Willm, A, Chassot, AA, Mendez-Otalvaro, EF, Sueur, B, Roßmann, K et al.. Light-induced analgesia provides a drug-free optical method for pain relief via activation of TRAAK k+ channels. Nat Commun. 2026;17 (1):620. doi: 10.1038/s41467-025-67819-w. PubMed PMID:41587988 PubMed Central PMC12834984.
- Luque-Fernández, V, Vanspauwen, SK, Landra-Willm, A, Arvedsen, E, Besquent, M, Sandoz, G et al.. An ankyrin G-binding motif mediates TRAAK periodic localization at axon initial segments of hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2024;121 (31):e2310120121. doi: 10.1073/pnas.2310120121. PubMed PMID:39058579 PubMed Central PMC11295008.
- Stockinger, F, Poc, P, Möhwald, A, Karch, S, Häfner, S, Alzheimer, C et al.. Multicolor, Cell-Impermeable, and High Affinity BACE1 Inhibitor Probes Enable Superior Endogenous Staining and Imaging of Single Molecules. J Med Chem. 2024;67 (12):10152-10167. doi: 10.1021/acs.jmedchem.4c00339. PubMed PMID:38842406 PubMed Central PMC11215771.
- Eria-Oliveira, AS, Folacci, M, Chassot, AA, Fedou, S, Thézé, N, Zabelskii, D et al.. Hijacking of internal calcium dynamics by intracellularly residing viral rhodopsins. Nat Commun. 2024;15 (1):65. doi: 10.1038/s41467-023-44548-6. PubMed PMID:38167346 PubMed Central PMC10761956.
- Landra-Willm, A, Karapurkar, A, Duveau, A, Chassot, AA, Esnault, L, Callejo, G et al.. A photoswitchable inhibitor of TREK channels controls pain in wild-type intact freely moving animals. Nat Commun. 2023;14 (1):1160. doi: 10.1038/s41467-023-36806-4. PubMed PMID:36859433 PubMed Central PMC9977718.
- Ávalos Prado, P, Chassot, AA, Landra-Willm, A, Sandoz, G. Regulation of two-pore-domain potassium TREK channels and their involvement in pain perception and migraine. Neurosci Lett. 2022;773 :136494. doi: 10.1016/j.neulet.2022.136494. PubMed PMID:35114333 .
- Häfner, S, Sandoz, G. Photopharmacological approaches for dissecting potassium channel physiology. Curr Opin Pharmacol. 2022;63 :102178. doi: 10.1016/j.coph.2021.12.005. PubMed PMID:35065384 .
- Ávalos Prado, P, Landra-Willm, A, Verkest, C, Ribera, A, Chassot, AA, Baron, A et al.. TREK channel activation suppresses migraine pain phenotype. iScience. 2021;24 (9):102961. doi: 10.1016/j.isci.2021.102961. PubMed PMID:34458705 PubMed Central PMC8379698.
- Gutzeit, VA, Acosta-Ruiz, A, Munguba, H, Häfner, S, Landra-Willm, A, Mathes, B et al.. A fine-tuned azobenzene for enhanced photopharmacology in vivo. Cell Chem Biol. 2021;28 (11):1648-1663.e16. doi: 10.1016/j.chembiol.2021.02.020. PubMed PMID:33735619 PubMed Central PMC8435545.
- Ávalos Prado, P, Häfner, S, Comoglio, Y, Wdziekonski, B, Duranton, C, Attali, B et al.. KCNE1 is an auxiliary subunit of two distinct ion channel superfamilies. Cell. 2021;184 (2):534-544.e11. doi: 10.1016/j.cell.2020.11.047. PubMed PMID:33373586 .
- Verkest, C, Häfner, S, Ávalos Prado, P, Baron, A, Sandoz, G. Migraine and Two-Pore-Domain Potassium Channels. Neuroscientist. 2021;27 (3):268-284. doi: 10.1177/1073858420940949. PubMed PMID:32715910 .
- Ávalos Prado, P, Sandoz, G. TREK for High-Speed and High-Frequency Conduction through the Axon. Neuron. 2019;104 (5):831-833. doi: 10.1016/j.neuron.2019.11.015. PubMed PMID:31805261 .
- Royal, P, Sandoz, G. [Ion channels and mechanisms of inherited disease transmission causing migraine]. Med Sci (Paris). 2019;35 (8-9):608-610. doi: 10.1051/medsci/2019121. PubMed PMID:31539490 .
- Royal, P, Ávalos Prado, P, Wdziekonski, B, Sandoz, G. [Two-pore-domain potassium channels and molecular mechanisms underlying migraine]. Biol Aujourdhui. 2019;213 (1-2):51-57. doi: 10.1051/jbio/2019020. PubMed PMID:31274103 .
- Royal, P, Andres-Bilbe, A, Ávalos Prado, P, Verkest, C, Wdziekonski, B, Schaub, S et al.. Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK. Neuron. 2019;101 (2):232-245.e6. doi: 10.1016/j.neuron.2018.11.039. PubMed PMID:30573346 .
- Rayaprolu, V, Royal, P, Stengel, K, Sandoz, G, Kohout, SC. Dimerization of the voltage-sensing phosphatase controls its voltage-sensing and catalytic activity. J Gen Physiol. 2018;150 (5):683-696. doi: 10.1085/jgp.201812064. PubMed PMID:29695412 PubMed Central PMC5940254.
- Song, OR, Kim, HB, Jouny, S, Ricard, I, Vandeputte, A, Deboosere, N et al.. A Bacterial Toxin with Analgesic Properties: Hyperpolarization of DRG Neurons by Mycolactone. Toxins (Basel). 2017;9 (7):. doi: 10.3390/toxins9070227. PubMed PMID:28718822 PubMed Central PMC5535174.
- Levitz, J, Royal, P, Comoglio, Y, Wdziekonski, B, Schaub, S, Clemens, DM et al.. Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc Natl Acad Sci U S A. 2016;113 (15):4194-9. doi: 10.1073/pnas.1522459113. PubMed PMID:27035963 PubMed Central PMC4839437.
- Song, OR, Marion, E, Comoglio, Y, Babonneau, J, Guerineau, N, Sandoz, G et al.. [Mycolactone: the amazing analgesic mycobacterial toxin]. Med Sci (Paris). 2016;32 (2):156-8. doi: 10.1051/medsci/20163202007. PubMed PMID:26936171 .
- Harb, K, Magrinelli, E, Nicolas, CS, Lukianets, N, Frangeul, L, Pietri, M et al.. Area-specific development of distinct projection neuron subclasses is regulated by postnatal epigenetic modifications. Elife. 2016;5 :e09531. doi: 10.7554/eLife.09531. PubMed PMID:26814051 PubMed Central PMC4744182.
2024 - Prix Fondation Simone et Cino del Duca de l'Académie des Sciences
2016 - Travel Grant award for SFN - INSCOPIX
2012 - Laboratory of Excellence LABEX awarded
2012 - ATIP-AVENIR award
2009 - Fulbright award
2009 - Philippe Foundation awarded
2007 - Prix Jean-Louis Parrot - French Society for Promotion of Sciences (AFAS)

Light-Induced Analgesia: a promising discovery for animal welfare
Read More

Guillaume Sandoz receives prestigious grant from the Simone and Cino Del Duca Foundation
Read More

Guillaume Sandoz’s team highlighted in Nice Matin for its recent advances in optogenetics!
Read More
Sandoz team highlighted by Inserm in “scientific advances of 2021”
Read More
Best PhD thesis 2020 award for Pablo AVALOS PRADO from iBV
Read More
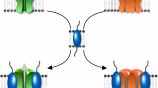
Challenging the protein complexes dogma
Read More
Discovery of novel mechanisms that cause migraines
Read More
iBV - Institut de Biologie Valrose
"Sciences Naturelles"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
