PLoS Biol. 2020 Nov 17;18(11):e3000902. doi: 10.1371/journal.pbio.3000902. eCollection 2020 Nov.
Glenda Evangelina Comai 1 2, Markéta Tesařová 3, Valérie Dupé 4, Muriel Rhinn 5, Pedro Vallecillo-García 6, Fabio da Silva 7 8, Betty Feret 5, Katherine Exelby 9, Pascal Dollé 5, Leif Carlsson 10, Brian Pryce 11, François Spitz 12, Sigmar Stricker 6, Tomáš Zikmund 3, Jozef Kaiser 3, James Briscoe 9, Andreas Schedl 7, Norbert B Ghyselinck 5, Ronen Schweitzer 11, Shahragim Tajbakhsh 1 2
Affiliations
1 Stem Cells & Development Unit, Institut Pasteur, Paris, France.
2 CNRS UMR 3738, Institut Pasteur, Paris, France.
3 Central European Institute of Technology, Brno University of Technology, Brno, Czech Republic.
4 Université de Rennes, CNRS, IGDR, Rennes, France.
5 IGBMC-Institut de Génétique et de Biologie Moleculaire et Cellulaire, Illkirch, France.
6 Institute for Chemistry and Biochemistry, Freie Universität Berlin, Berlin, Germany.
7 Université Côte d’Azur, INSERM, CNRS, iBV, Nice, France.
8 Division of Molecular Embryology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
9 The Francis Crick Institute, London, United Kingdom.
10 Umeå Center for Molecular Medicine, Umeå University, Umeå, Sweden.
11 Research Division, Shriners Hospital for Children, Portland, United States of America.
12 Genomics of Animal Development Unit, Institut Pasteur, Paris, France.
Abstract
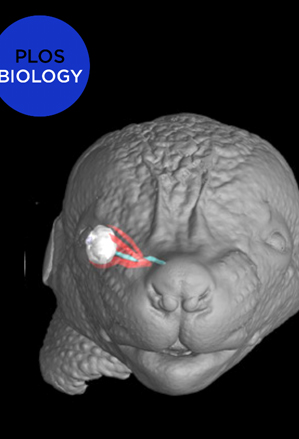
Coordinated development of muscles, tendons, and their attachment sites ensures emergence of functional musculoskeletal units that are adapted to diverse anatomical demands among different species. How these different tissues are patterned and functionally assembled during embryogenesis is poorly understood. Here, we investigated the morphogenesis of extraocular muscles (EOMs), an evolutionary conserved cranial muscle group that is crucial for the coordinated movement of the eyeballs and for visual acuity. By means of lineage analysis, we redefined the cellular origins of periocular connective tissues interacting with the EOMs, which do not arise exclusively from neural crest mesenchyme as previously thought. Using 3D imaging approaches, we established an integrative blueprint for the EOM functional unit. By doing so, we identified a developmental time window in which individual EOMs emerge from a unique muscle anlage and establish insertions in the sclera, which sets these muscles apart from classical muscle-to-bone type of insertions. Further, we demonstrate that the eyeballs are a source of diffusible all-trans retinoic acid (ATRA) that allow their targeting by the EOMs in a temporal and dose-dependent manner. Using genetically modified mice and inhibitor treatments, we find that endogenous local variations in the concentration of retinoids contribute to the establishment of tendon condensations and attachment sites that precede the initiation of muscle patterning. Collectively, our results highlight how global and site-specific programs are deployed for the assembly of muscle functional units with precise definition of muscle shapes and topographical wiring of their tendon attachments.
PMID: 33201874
DOI: 10.1371/journal.pbio.3000902