
Maximilian FÜRTHAUER
Membrane trafficking and developmental signalling in animal development
Main interests
- Delta/Notch signaling and neuro-epithelial polarity
- Developmental functions of the ESCRT machinery
- Extracellular vesicles in animal development
- Establishing Left/Right asymmetry: from cell polarity to organismal laterality
Scientific Questions
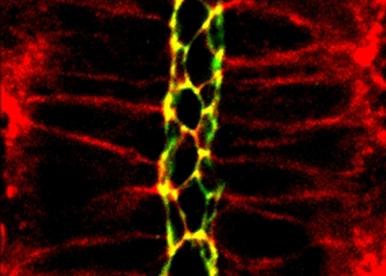
Cellular membranes are essential for the functional compartmentalization of the organism. Within cells, they enable the formation of specialized functional domains at the level of the plasma membrane or intracellular organelles. At the level of the organism, they provide interfaces that delimit the organism from the outside world and individual cells within tissues. Last not least, an increasing body of evidence suggests that membrane-bound extracellular vesicles play an important role in animal development and physiology. The overall aim of our research is to understand how the formation, function and dynamic remodeling of membrane-bound compartments governs the localization and activity of signaling molecules in animal development.
Our Strategy
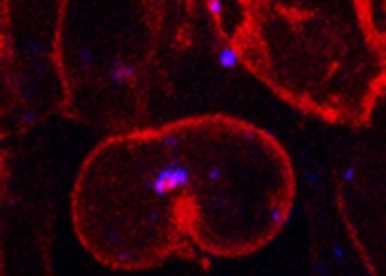
Our group has been using two complementary model systems, zebrafish and Drosophila to address two major questions:
First, how does endocytic membrane trafficking regulate developmental signalling?
Delta ligands and Notch receptors define a signaling pathway of major importance for development and disease. Notch receptor activation is dependent on Delta ligand endocytosis, but the actual mechanistic reason for this requirement is not fully understood. We established a novel assay to track endogenous signaling molecules in vivo and identified the asymmetric segregation of Delta-containing endosomes as a novel mechanism to promote directional Notch signaling in asymmetrically dividing neural precursor cells (Coumailleau et al 2009, Kressmann et al, 2015).
Second, how does a major membrane remodeling machinery control embryonic development?
The Endosomal Sorting Complex Required for Transport (ESCRT) is an evolutionarily conserved membrane-remodeling machinery that was initially discovered for its function in the endocytic regulation of growth factor signaling. Our work suggests that another important function of the ESCRT machinery is to promote the virus-like cell surface budding of extracellular vesicles that ensure the transport of developmental signaling molecules such as the major developmental morphogen Hedgehog (Matusek et al, 2014, Juan and Fürthauer, 2018).
Research Aims
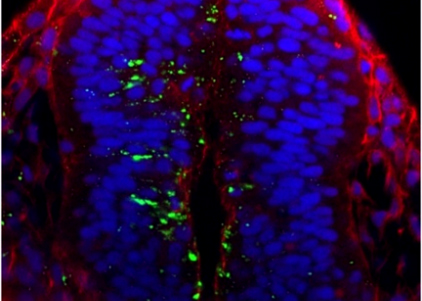
Notch receptor activation has been proposed to rely on the apico-basal transport of endocytosed Delta ligand molecules. Through the development of novel live imaging assays we are now able to visualize this process in vivo, in the context of the development of the zebrafish neural tube. Using a combination of genetic and live imaging approaches, we are aiming to dissect the functional links between neuro-epithelial polarity and Delta/Notch signalling.
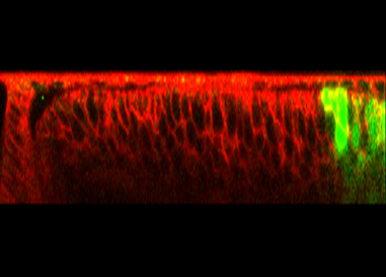
The Endosomal Sorting Complex Required for Transport (ESCRT) is well known for its essential role in the endocytic regulation of growth factor signaling. In contrast, how other ESCRT-dependent processes such as the secretion of extracellular vesicles contribute to animal development remains largely to be understood. A second major aim of our work is to characterize novel functions of this major membrane trafficking machinery in animal development.
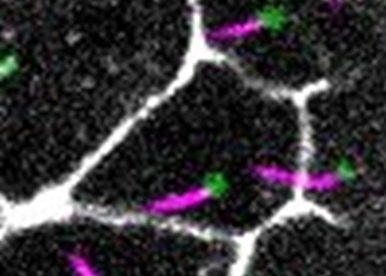
In contrast to intracellular endosomes, Extracellular Vesicles (EVs) cannot use cytoskeleton-dependent molecular motors for their movement. We study how oriented motile cilia are used to create directional fluid flows in the extracellular space to drive EV transport. We have shown that Myosin1D controls motile cilia orientation in the zebrafish left-right organizer, identifying this actin-binding motor protein as a conserved regulator of animal left-right asymmetry (Juan et al, 2018).
PreDocs
 GUEYE Mansour - +33 R
GUEYE Mansour - +33 R
Engineers & Technicians
 POLES Sophie - +33 489150836
POLES Sophie - +33 489150836 SALMY Farid - +33 R
SALMY Farid - +33 R FAYOUX Anne - +33 R
FAYOUX Anne - +33 R
Recent publications
- Kurup, AJ, Bailet, F, Fürthauer, M. Myosin1G promotes Nodal signaling to control zebrafish left-right asymmetry. Nat Commun. 2024;15 (1):6547. doi: 10.1038/s41467-024-50868-y. PubMed PMID:39095343 PubMed Central PMC11297164.
- Jeong, I, Andreassen, SN, Hoang, L, Poulain, M, Seo, Y, Park, HC et al.. The evolutionarily conserved choroid plexus contributes to the homeostasis of brain ventricles in zebrafish. Cell Rep. 2024;43 (6):114331. doi: 10.1016/j.celrep.2024.114331. PubMed PMID:38843394 .
- Saraswathy, VM, Kurup, AJ, Sharma, P, Polès, S, Poulain, M, Fürthauer, M et al.. The E3 ubiquitin ligase mindbomb1 controls planar cell polarity-dependent convergent extension movements during zebrafish gastrulation. Elife. 2022;11 :. doi: 10.7554/eLife.71928. PubMed PMID:35142609 PubMed Central PMC8937233.
- Sharma, P, Saraswathy, VM, Xiang, L, Fürthauer, M. Notch-mediated inhibition of neurogenesis is required for zebrafish spinal cord morphogenesis. Sci Rep. 2019;9 (1):9958. doi: 10.1038/s41598-019-46067-1. PubMed PMID:31292468 PubMed Central PMC6620349.
- Matusek, T, Thérond, P, Fürthauer, M. Functional Analysis of ESCRT-Positive Extracellular Vesicles in the Drosophila Wing Imaginal Disc. Methods Mol Biol. 2019;1998 :31-47. doi: 10.1007/978-1-4939-9492-2_3. PubMed PMID:31250292 .
- Juan, T, Géminard, C, Coutelis, JB, Cerezo, D, Polès, S, Noselli, S et al.. Myosin1D is an evolutionarily conserved regulator of animal left-right asymmetry. Nat Commun. 2018;9 (1):1942. doi: 10.1038/s41467-018-04284-8. PubMed PMID:29769531 PubMed Central PMC5955935.
- Fürthauer, M. The ESCRT machinery: When function follows form. Semin Cell Dev Biol. 2018;74 :1-3. doi: 10.1016/j.semcdb.2017.11.003. PubMed PMID:29113869 .
- Juan, T, Fürthauer, M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74 :66-77. doi: 10.1016/j.semcdb.2017.08.022. PubMed PMID:28807885 .
- Mamińska, A, Bartosik, A, Banach-Orłowska, M, Pilecka, I, Jastrzębski, K, Zdżalik-Bielecka, D et al.. ESCRT proteins restrict constitutive NF-κB signaling by trafficking cytokine receptors. Sci Signal. 2016;9 (411):ra8. doi: 10.1126/scisignal.aad0848. PubMed PMID:26787452 .
- Juan, T, Fürthauer, M. [The ESCRT complex: from endosomal transport to the development of multicellular organisms]. Biol Aujourdhui. 2015;209 (1):111-24. doi: 10.1051/jbio/2015009. PubMed PMID:26115716 .
- Loubéry, S, Seum, C, Moraleda, A, Daeden, A, Fürthauer, M, Gonzalez-Gaitan, M et al.. Uninflatable and Notch Control the Targeting of Sara Endosomes during Asymmetric Division. Curr Biol. 2015;25 (6):817-818. doi: 10.1016/j.cub.2015.02.053. PubMed PMID:29665399 .
- Kressmann, S, Campos, C, Castanon, I, Fürthauer, M, González-Gaitán, M. Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat Cell Biol. 2015;17 (3):333-9. doi: 10.1038/ncb3119. PubMed PMID:25706234 .
- Matusek, T, Wendler, F, Polès, S, Pizette, S, D'Angelo, G, Fürthauer, M et al.. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516 (7529):99-103. doi: 10.1038/nature13847. PubMed PMID:25471885 .
- Loubéry, S, Seum, C, Moraleda, A, Daeden, A, Fürthauer, M, Gonzalez-Gaitan, M et al.. Uninflatable and Notch control the targeting of Sara endosomes during asymmetric division. Curr Biol. 2014;24 (18):2142-2148. doi: 10.1016/j.cub.2014.07.054. PubMed PMID:25155514 .
- Fürthauer, M, Smythe, E. Systems dynamics in endocytosis. Traffic. 2014;15 (3):338-46. doi: 10.1111/tra.12147. PubMed PMID:24405722 .
- Cavodeassi, F, Del Bene, F, Fürthauer, M, Grabher, C, Herzog, W, Lehtonen, S et al.. Report of the Second European Zebrafish Principal Investigator Meeting in Karlsruhe, Germany, March 21-24, 2012. Zebrafish. 2013;10 (1):119-23. doi: 10.1089/zeb.2012.0829. PubMed PMID:23530760 .
- Fürthauer, M, González-Gaitán, M. Endocytosis and mitosis: a two-way relationship. Cell Cycle. 2009;8 (20):3311-8. doi: 10.4161/cc.8.20.9700. PubMed PMID:19770584 .
- Fürthauer, M, González-Gaitán, M. Endocytosis, asymmetric cell division, stem cells and cancer: unus pro omnibus, omnes pro uno. Mol Oncol. 2009;3 (4):339-53. doi: 10.1016/j.molonc.2009.05.006. PubMed PMID:19581131 PubMed Central PMC5527947.
- Fürthauer, M, González-Gaitán, M. Tales of 1001 functions: the multiple roles of membrane trafficking in development. Traffic. 2009;10 (7):781-2. doi: 10.1111/j.1600-0854.2009.00931.x. PubMed PMID:19570190 .
- Fürthauer, M, González-Gaitán, M. Endocytic regulation of notch signalling during development. Traffic. 2009;10 (7):792-802. doi: 10.1111/j.1600-0854.2009.00914.x. PubMed PMID:19416471 .
2011 - HFSP Career Development Award
2010 - ATIP/Avenir Junior Group Leeder Programme
2005 - Long Term Postdoctoral Fellowship, HFSP
2004 - Postdoctoral fellowship, EMBO

A new molecular player makes hearts go left
Read More

UCA Annual Award Ceremony 2019: 4 iBV members recognized this year !
Read More

Thomas Juan, PhD student of Maximilian Fürthauer’s team at iBV, winner of the Bettencourt Prize for Young Researchers 2019
Read More

“Best PhD thesis 2017” award ceremony
Read More
PhD Prize awarded to Thomas JUAN from iBV !
Read More
iBV - Institut de Biologie Valrose
"Sciences Naturelles"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
