
Stéphane NOSELLI
Epithelial Morphogenesis and left-right asymmetry in Drosophila
Main interests
- Mechanisms controlling Visceral and Brain Left-Right Asymmetry
- Role of the conserved Myosin1D and of the Actin Cytoskeleton in LR Symmetry Breaking
- Origin, Propagation and Evolution of Biological Chirality
- Signaling Pathways controlling Collective Cell Migration
Scientific Questions
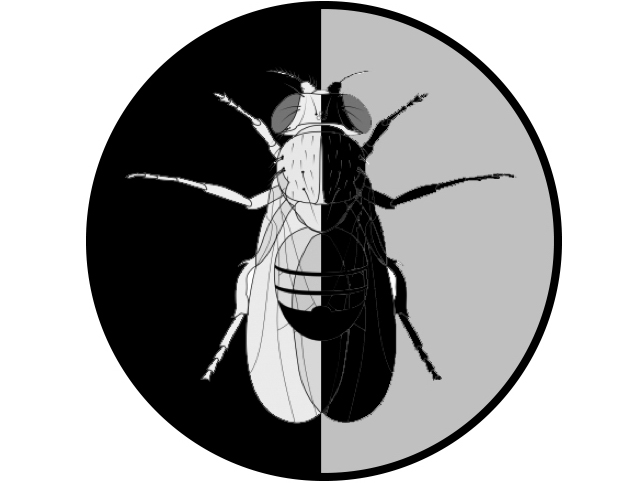
Emergence of Asymmetry from an initially symmetrical state is a universal transition in Nature. Living organisms show striking asymmetries at all scales (molecular, cellular, tissular, organismal) and one of their fundamental features lies in their assembly from homochiral molecular components. Whether the macroscopic asymmetries of living systems are directly related to their molecular chirality remains an open question. We study Left-Right Asymmetry in Drosophila to characterize the molecular basis of laterality and address the origin, propagation and evolution of symmetry breaking.
Tissue morphogenesis relies on another fundamental transition, the Immotile-to-Motile Transition, leading individual cells or groups of cells to undergo migration. The acquisition of motility by cancer cells leads them to become metastatic or invasive. Drosophila Border Cells represent a powerful paradigm to study collective cell migration in vivo. Using this system, we characterize the role of new genes and conserved signaling pathways controlling motility.
Our Strategy
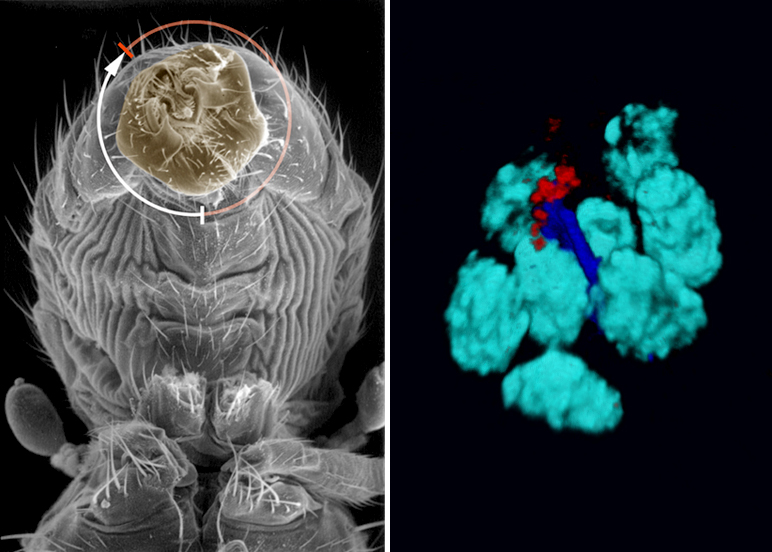
Our research program aims at better understanding the mechanisms underlying symmetry breaking, and how this information is propagated to target cells and organs. We are also trying to understand how asymmetry can cross organizational layers to become multiscale (from molecular to behavioral) and whether these fundamental mechanisms are conserved during evolution. To these goals, we initiated the study of LR asymmetry in Drosophila. Our previous work identified Myosin1D as a unique situs inversus gene, regulating visceral LR asymmetry through cell death, E-Cadherin, HOX genes and the conserved PCP pathways. More recently, we have shown that Myo1D orthologs control LR asymmetry in both xenopus and zebrafish, revealing Myo1D as the first unifying principle of laterality across phyla.
Our current work aims at identifying new genes/pathways and concepts controlling asymmetry and motility, using multiscale approaches combining biochemistry, genetics, cell biology and modeling. More specifically, we design large-scale genetic and molecular screens to i) identify new genes controlling visceral LR asymmetry, ii) establish Drosophila as a new paradigm to study brain asymmetry and iii) identify new genes involved in the control of cell motility in vivo.
Research Aims
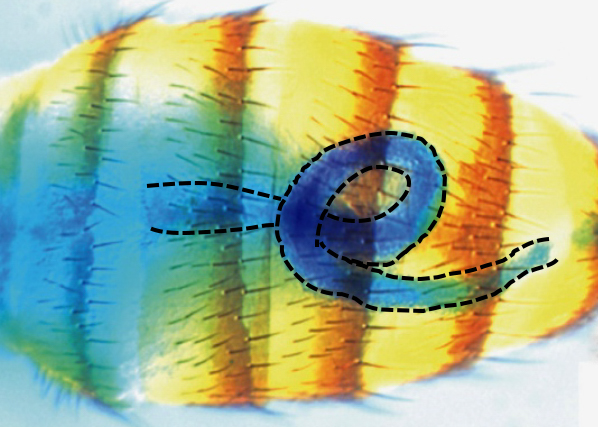
VISCERAL LR ASYMMETRY is controlled by the conserved Myosin1D protein which is active in multiple and independent LR organizers. In these, Myo1D sets and propagates asymmetry to individual organs (genitalia, hindgut, etc.), each of them having their own morphogenetic program. We are currently deciphering the core asymmetry pathway, the mode(s) of information propagation as well as downstream morphogenetic programs by i) characterizing new genes involved in the Myo1D pathway, ii) studying the interaction between the Myo1D and PCP pathways, iii) determining the role of the actin cytoskeleton in the process.
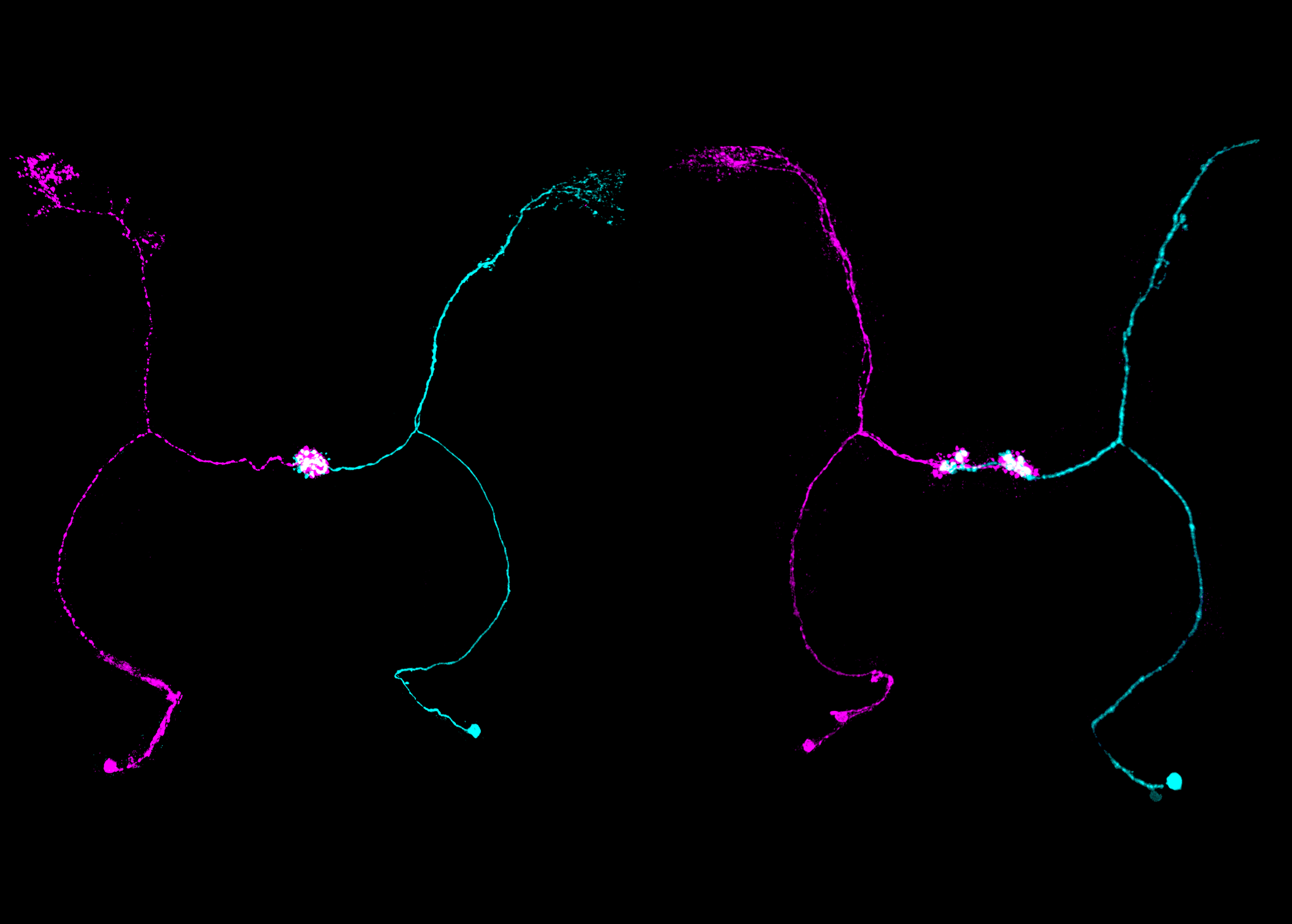
BRAIN LR ASYMMETRY is essential for a number of cognitive and behavioral processes, yet the mechanisms controlling nervous system asymmetry are poorly understood. Of note, brain and visceral asymmetry are mostly controlled by specific programs. We are currently initiating the study of nervous system LR asymmetry in Drosophila through large-scale genetic screening and functional characterization. Our recent results allowed the identification of the first genes and mechanisms underlying brain asymmetry in flies. The role of gene orthologs will be studied in the vertebrate nervous system.
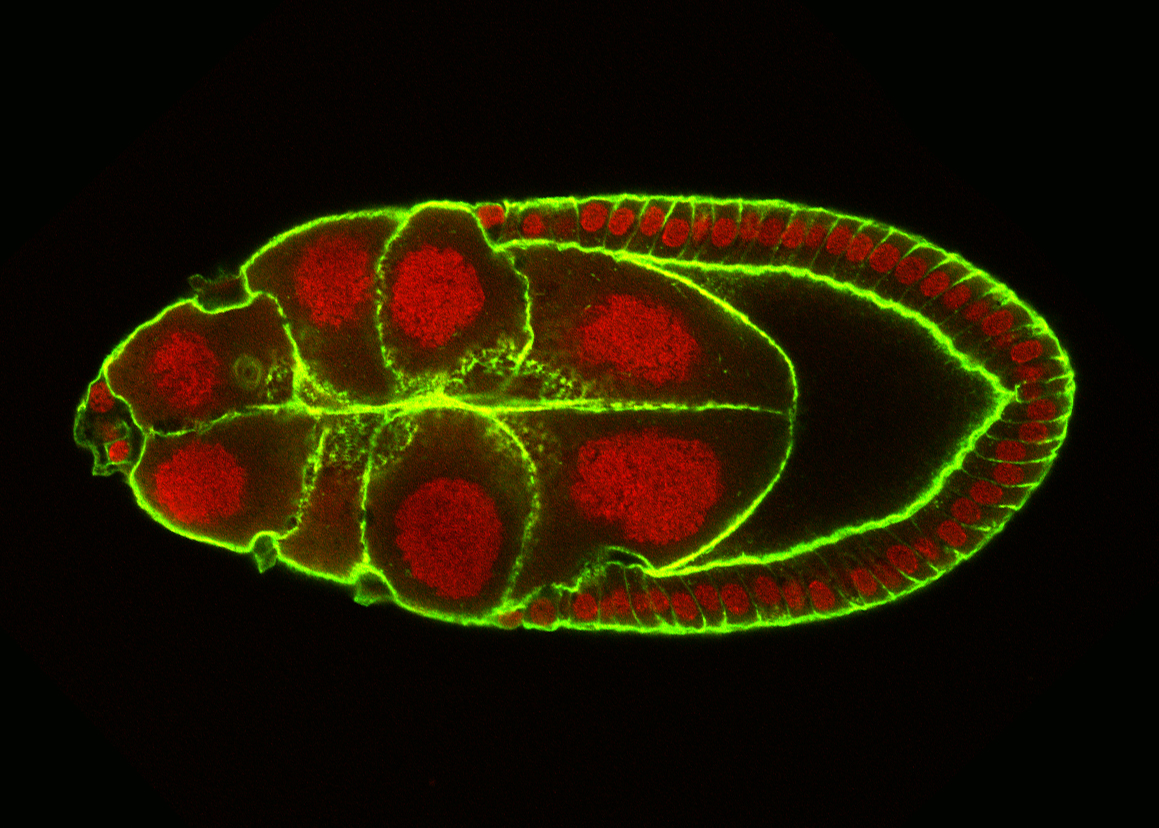
CELL MOTILITY is a complex, multistep process involving communication with neighboring tissues and the environment. Transition from an immotile to a motile phenotype depends on the regulation of cell adhesion, polarity, growth and signalling. To understand how cohorts of cells migrate in a coordinated manner, we are identifying and characterizing new regulators of Border Cell migration during Drosophila oogenesis.
Researchers
 GHIGLIONE Christian - +33 489150751
GHIGLIONE Christian - +33 489150751 VAN DE BOR Véronique - +33 489150753
VAN DE BOR Véronique - +33 489150753
PreDocs
 RAVEL Nils - +33 489150752
RAVEL Nils - +33 489150752 MOUCHET Coralie - +33 R
MOUCHET Coralie - +33 R
Engineers & Technicians
 CEREZO Delphine - +33 489150752
CEREZO Delphine - +33 489150752 LAPRAZ François - +33 489150753
LAPRAZ François - +33 489150753 BOUTRES Céline - +33 489150753
BOUTRES Céline - +33 489150753
Recent publications
- Lapraz, F, Fixary-Schuster, C, Noselli, S. Brain bilateral asymmetry - insights from nematodes, zebrafish, and Drosophila. Trends Neurosci. 2024;47 (10):803-818. doi: 10.1016/j.tins.2024.08.003. PubMed PMID:39322499 .
- Fixary-Schuster, C, Lapraz, F, Noselli, S. [Netrin signaling pathway, memory and brain lateralization]. Med Sci (Paris). 2024;40 (2):139-142. doi: 10.1051/medsci/2023209. PubMed PMID:38411419 .
- Lapraz, F, Boutres, C, Fixary-Schuster, C, De Queiroz, BR, Plaçais, PY, Cerezo, D et al.. Asymmetric activity of NetrinB controls laterality of the Drosophila brain. Nat Commun. 2023;14 (1):1052. doi: 10.1038/s41467-023-36644-4. PubMed PMID:36828820 PubMed Central PMC9958012.
- Banreti, A, Bhattacharya, S, Wien, F, Matsuo, K, Réfrégiers, M, Meinert, C et al.. Biological effects of the loss of homochirality in a multicellular organism. Nat Commun. 2022;13 (1):7059. doi: 10.1038/s41467-022-34516-x. PubMed PMID:36400783 PubMed Central PMC9674851.
- Van De Bor, V, Loreau, V, Malbouyres, M, Cerezo, D, Placenti, A, Ruggiero, F et al.. A dynamic and mosaic basement membrane controls cell intercalation in Drosophila ovaries. Development. 2021;148 (4):. doi: 10.1242/dev.195511. PubMed PMID:33526583 .
- Chougule, A, Lapraz, F, Földi, I, Cerezo, D, Mihály, J, Noselli, S et al.. The Drosophila actin nucleator DAAM is essential for left-right asymmetry. PLoS Genet. 2020;16 (4):e1008758. doi: 10.1371/journal.pgen.1008758. PubMed PMID:32324733 PubMed Central PMC7200016.
- Rice, G, David, JR, Kamimura, Y, Masly, JP, Mcgregor, AP, Nagy, O et al.. A standardized nomenclature and atlas of the male terminalia of Drosophila melanogaster. Fly (Austin). 2019;13 (1-4):51-64. doi: 10.1080/19336934.2019.1653733. PubMed PMID:31401934 PubMed Central PMC6988887.
- Lebreton, G, Géminard, C, Lapraz, F, Pyrpassopoulos, S, Cerezo, D, Spéder, P et al.. Molecular to organismal chirality is induced by the conserved myosin 1D. Science. 2018;362 (6417):949-952. doi: 10.1126/science.aat8642. PubMed PMID:30467170 PubMed Central PMC6698710.
- Ghiglione, C, Jouandin, P, Cérézo, D, Noselli, S. The Drosophila insulin pathway controls Profilin expression and dynamic actin-rich protrusions during collective cell migration. Development. 2018;145 (14):. doi: 10.1242/dev.161117. PubMed PMID:29980565 .
- Juan, T, Géminard, C, Coutelis, JB, Cerezo, D, Polès, S, Noselli, S et al.. Myosin1D is an evolutionarily conserved regulator of animal left-right asymmetry. Nat Commun. 2018;9 (1):1942. doi: 10.1038/s41467-018-04284-8. PubMed PMID:29769531 PubMed Central PMC5955935.
- Tingler, M, Kurz, S, Maerker, M, Ott, T, Fuhl, F, Schweickert, A et al.. A Conserved Role of the Unconventional Myosin 1d in Laterality Determination. Curr Biol. 2018;28 (5):810-816.e3. doi: 10.1016/j.cub.2018.01.075. PubMed PMID:29478852 .
- Roumengous, S, Rousset, R, Noselli, S. Polycomb and Hox Genes Control JNK-Induced Remodeling of the Segment Boundary during Drosophila Morphogenesis. Cell Rep. 2017;19 (1):60-71. doi: 10.1016/j.celrep.2017.03.033. PubMed PMID:28380363 .
- Rousset, R, Carballès, F, Parassol, N, Schaub, S, Cérézo, D, Noselli, S et al.. Signalling crosstalk at the leading edge controls tissue closure dynamics in the Drosophila embryo. PLoS Genet. 2017;13 (2):e1006640. doi: 10.1371/journal.pgen.1006640. PubMed PMID:28231245 PubMed Central PMC5344535.
- Van De Bor, V, Zimniak, G, Papone, L, Cerezo, D, Malbouyres, M, Juan, T et al.. Companion Blood Cells Control Ovarian Stem Cell Niche Microenvironment and Homeostasis. Cell Rep. 2015;13 (3):546-560. doi: 10.1016/j.celrep.2015.09.008. PubMed PMID:26456819 .
- González-Morales, N, Géminard, C, Lebreton, G, Cerezo, D, Coutelis, JB, Noselli, S et al.. The Atypical Cadherin Dachsous Controls Left-Right Asymmetry in Drosophila. Dev Cell. 2015;33 (6):675-89. doi: 10.1016/j.devcel.2015.04.026. PubMed PMID:26073018 .
- Coutelis, JB, González-Morales, N, Géminard, C, Noselli, S. Diversity and convergence in the mechanisms establishing L/R asymmetry in metazoa. EMBO Rep. 2014;15 (9):926-37. doi: 10.15252/embr.201438972. PubMed PMID:25150102 PubMed Central PMC4198036.
- Jouandin, P, Ghiglione, C, Noselli, S. Starvation induces FoxO-dependent mitotic-to-endocycle switch pausing during Drosophila oogenesis. Development. 2014;141 (15):3013-21. doi: 10.1242/dev.108399. PubMed PMID:24993942 PubMed Central PMC6514422.
- Géminard, C, González-Morales, N, Coutelis, JB, Noselli, S. The myosin ID pathway and left-right asymmetry in Drosophila. Genesis. 2014;52 (6):471-80. doi: 10.1002/dvg.22763. PubMed PMID:24585718 .
- Parassol, N, Bienvenu, C, Boglio, C, Fiorucci, S, Cerezo, D, Yu, XM et al.. In vivo characterization of dynein-driven nanovectors using Drosophila oocytes. PLoS One. 2013;8 (12):e82908. doi: 10.1371/journal.pone.0082908. PubMed PMID:24349395 PubMed Central PMC3861458.
- Coutelis, JB, Géminard, C, Spéder, P, Suzanne, M, Petzoldt, AG, Noselli, S et al.. Drosophila left/right asymmetry establishment is controlled by the Hox gene abdominal-B. Dev Cell. 2013;24 (1):89-97. doi: 10.1016/j.devcel.2012.11.013. PubMed PMID:23328400 .
2018 - Elected Member of Academia Europaea
2014 - CNRS Research Director - Exceptional Class
2014 - EMBO Member - EMBO
2013 - Grand Prix Mottart - Académie des Sciences
2008 - Excellence Award (Prime d'excellence Scientifique) - CNRS
2008 - Team FRM
2008 - Silver Medal - CNRS
2007 - "Grandes Avancées Françaises en Biologie" - French Academy of Sciences
2001 - Young Investigator Program - EMBO
1999 - ATIP Developmental Biology - CNRS
1998 - Bronze Medal - CNRS

Complete decoding of brain connections in Drosophila
Read More

Congratulations to Yasmine Neirijnck and Agnes Banreti for their INSERM permanent positions!
Read More

UCA annual Award Ceremony: 7 iBV members recognised for their scientific contributions
Read More
The origins of asymmetry: A protein that makes you do the twist
Read More

Stéphane NOSELLI is elected Member of Academia Europaea
Read More
iBV - Institut de Biologie Valrose
"Centre de Biochimie"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
