
Anne-Odile HUEBER
Death receptors signalling and cancer therapy
Main interests
- death receptors in cancer biology and anti-cancer therapies
- Fas (TNFRSF6/CD95) multi-signaling
- control of life and death of cancer cells
Scientific Questions
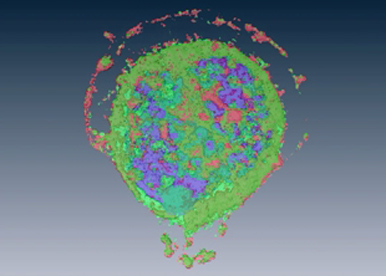
The effectiveness of current cancer therapies is limited by tumor resistance to chemotherapy and targeted therapies. Combinatorial multi-pathway targeted therapies and the use of predictive biomarkers to stratify patients who may benefit from therapies are key strategies to overcome this therapeutic challenge. They require identification of molecular targets and biomarkers and thus the understanding of molecular basis of signaling pathways that can be targeted in order to reconstitute programmed cell death (apoptosis) and prevent uncontrolled proliferation of cancer cells. In this context, understanding the molecular basis of the imbalance between cell death and cell survival acquired by cancer cells might lead to more efficient and targeted anti-cancer therapy and represents the main goal of our team. Amongst the proteins able to regulate both survival and death functions of the cells are the members of the TNF receptor (TNFR) superfamily.
Our Strategy
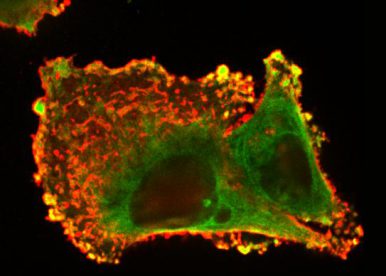
The multi-modal signaling in many cancer cell types, including colon, breast, and glioblastoma, has been demonstrated for the prototype of the TNFR superfamily, the receptor Fas (TNFRSF6/CD95/APO-1), so far considered a “purely” pro-apoptotic receptor. As Fas activates both cell death and survival, it is a good candidate for targeted therapies promoting or preventing cancer depending on cellular context. Our main recent research accomplishments concern the description of Fas multi-signaling regulation in cancer and its application in cancer therapy. Our work significantly contributed to a clearer understanding of how the Fas signaling is shifted from tumor-suppressing to tumor-driving mode, as well as presented the means to appropriately take control of the duality of Fas signaling in different pathological contexts in order to increase therapeutic success. In continuation of our research interest, we aim to achieve a deeper understanding in the versatility of Fas signaling and to clinically apply the knowledge in colorectal cancer (CRC) therapy. We will dissect the control of Fas signaling in the level of protein modifications, membrane receptor crosstalk, and multi-cellular regulation with the main aim to address the outstanding questions in the field of TNF receptor signaling and cancer cell biology: How does Fas multi-signaling influence CRC cellular functions and behavior and the CRC progression? And how can we use this information to the benefit of anti-cancer therapy?
Research Aims
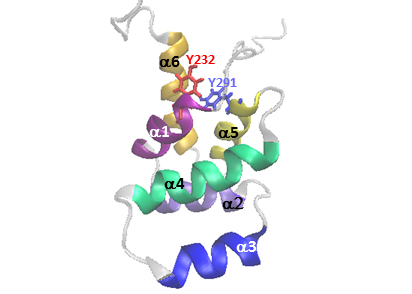
Influences of Fas pro-survival signal in the control of cancer-promoting functions of the EGFR: Our data reveal a tight relationship and compensation between Fas and ErbB family signaling as well as the indispensable role of Fas in the signaling of major drug targets, EGFR and ErbB2. We aim at elucidating the molecular machinery that controls the dynamic membrane organization of the Fas/ErbB survival signaling hub in different CRC contexts and their functional significance.
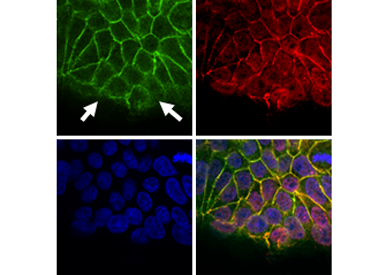
Regulation of Fas signaling by cell polarity: We hypothesize that apico-basal polarity is a crucial regulator of Fas signaling. We address the following questions: what is the exact role of cell polarity molecules, and the respective cell-cell adhesion structures, in Fas cell death signaling? Does the localization of the receptor influence its signaling capacity? Do the loss of polarity and the oncogene activation cooperate to switch Fas cell death signaling into the pro-tumoral signalling?
Regulation of T cell activation by the Fas non-apoptotic signaling: recent data support the role of the inhibitory function of Fas engagement by its membrane-bound ligand in TCR signaling. We then aim to dissect the molecular interplay of the TCR and Fas pathways at the membrane-proximal level during the activation of T cells. We believe our data will provide important new insights into the mechanism of primary immune response initiation, which plays a critical role in anti-cancer immunity.
Researchers
 ROSSIN Aurélie - +33 489150786
ROSSIN Aurélie - +33 489150786 VAN OBBERGHEN-SCHILLING Ellen - +33 489150790
VAN OBBERGHEN-SCHILLING Ellen - +33 489150790 GAGNOUX Laurent - +33 489150786
GAGNOUX Laurent - +33 489150786 BOUILHOL Emmanuel - +33 489150791
BOUILHOL Emmanuel - +33 489150791
Clinical Researchers
 GUIGAY Joël - +33 492031503
GUIGAY Joël - +33 492031503 BOZEC Alexandre - +33 R
BOZEC Alexandre - +33 R CULIE Damien - +33 R
CULIE Damien - +33 R
Recent Publications
- Grataitong, K, Huault, S, Chotwiwatthanakun, C, Jariyapong, P, Thongsum, O, Chawiwithaya, C et al.. Chimeric virus-like particles (VLPs) designed from shrimp nodavirus (MrNV) capsid protein specifically target EGFR-positive human colorectal cancer cells. Sci Rep. 2021;11 (1):16579. doi: 10.1038/s41598-021-95891-x. PubMed PMID:34400669 PubMed Central PMC8367941.
- González, R, Rodríguez-Hernández, MA, Negrete, M, Ranguelova, K, Rossin, A, Choya-Foces, C et al.. Downregulation of thioredoxin-1-dependent CD95 S-nitrosation by Sorafenib reduces liver cancer. Redox Biol. 2020;34 :101528. doi: 10.1016/j.redox.2020.101528. PubMed PMID:32388267 PubMed Central PMC7210585.
- Gagnoux-Palacios, L, Hueber, AO. [Cell death for maintaining epithelial homeostasis]. Med Sci (Paris). 2019;35 (11):830-833. doi: 10.1051/medsci/2019164. PubMed PMID:31845871 .
- Rossin, A, Miloro, G, Hueber, AO. TRAIL and FasL Functions in Cancer and Autoimmune Diseases: Towards an Increasing Complexity. Cancers (Basel). 2019;11 (5):. doi: 10.3390/cancers11050639. PubMed PMID:31072029 PubMed Central PMC6563024.
- Gagnoux-Palacios, L, Awina, H, Audebert, S, Rossin, A, Mondin, M, Borgese, F et al.. Cell polarity and adherens junction formation inhibit epithelial Fas cell death receptor signaling. J Cell Biol. 2018;217 (11):3839-3852. doi: 10.1083/jcb.201805071. PubMed PMID:30242034 PubMed Central PMC6219722.
- Ta, NL, Chakrabandhu, K, Huault, S, Hueber, AO. The tyrosine phosphorylated pro-survival form of Fas intensifies the EGF-induced signal in colorectal cancer cells through the nuclear EGFR/STAT3-mediated pathway. Sci Rep. 2018;8 (1):12424. doi: 10.1038/s41598-018-30804-z. PubMed PMID:30127519 PubMed Central PMC6102278.
- Hancz, D, Szabo, A, Molnar, T, Varga, Z, Hancz, A, Gregus, A et al.. Flagellin increases death receptor-mediated cell death in a RIP1-dependent manner. Immunol Lett. 2018;193 :42-50. doi: 10.1016/j.imlet.2017.11.007. PubMed PMID:29175315 .
- Rossin, A, Lounnas, N, Durivault, J, Miloro, G, Gagnoux-Palacios, L, Hueber, AO et al.. The Btk-dependent PIP5K1γ lipid kinase activation by Fas counteracts FasL-induced cell death. Apoptosis. 2017;22 (11):1344-1352. doi: 10.1007/s10495-017-1415-x. PubMed PMID:28879546 .
- Rossin, A, Hueber, AO. Detection of S-Acylated CD95 by Acyl-Biotin Exchange. Methods Mol Biol. 2017;1557 :189-198. doi: 10.1007/978-1-4939-6780-3_17. PubMed PMID:28078593 .
- Chakrabandhu, K, Huault, S, Hueber, AO. Site-Specific Detection of Tyrosine Phosphorylated CD95 Following Protein Separation by Conventional and Phospho-Protein Affinity SDS-PAGE. Methods Mol Biol. 2017;1557 :173-188. doi: 10.1007/978-1-4939-6780-3_16. PubMed PMID:28078592 .
- Chakrabandhu, K, Hueber, AO. Fas Versatile Signaling and Beyond: Pivotal Role of Tyrosine Phosphorylation in Context-Dependent Signaling and Diseases. Front Immunol. 2016;7 :429. doi: 10.3389/fimmu.2016.00429. PubMed PMID:27799932 PubMed Central PMC5066474.
- Chakrabandhu, K, Huault, S, Durivault, J, Lang, K, Ta Ngoc, L, Bole, A et al.. An Evolution-Guided Analysis Reveals a Multi-Signaling Regulation of Fas by Tyrosine Phosphorylation and its Implication in Human Cancers. PLoS Biol. 2016;14 (3):e1002401. doi: 10.1371/journal.pbio.1002401. PubMed PMID:26942442 PubMed Central PMC4778973.
- Wang, F, Weng, H, Quon, MJ, Yu, J, Wang, JY, Hueber, AO et al.. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy. Am J Physiol Endocrinol Metab. 2015;309 (10):E861-73. doi: 10.1152/ajpendo.00215.2015. PubMed PMID:26419589 PubMed Central PMC4652069.
- Dorvignit, D, García-Martínez, L, Rossin, A, Sosa, K, Viera, J, Hernández, T et al.. Antitumor and cytotoxic properties of a humanized antibody specific for the GM3(Neu5Gc) ganglioside. Immunobiology. 2015;220 (12):1343-50. doi: 10.1016/j.imbio.2015.07.008. PubMed PMID:26224247 .
- Andersen, DS, Colombani, J, Palmerini, V, Chakrabandhu, K, Boone, E, Röthlisberger, M et al.. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature. 2015;522 (7557):482-6. doi: 10.1038/nature14298. PubMed PMID:25874673 .
- Zhu, L, Derijard, B, Chakrabandhu, K, Wang, BS, Chen, HZ, Hueber, AO et al.. Synergism of PI3K/Akt inhibition and Fas activation on colon cancer cell death. Cancer Lett. 2014;354 (2):355-64. doi: 10.1016/j.canlet.2014.08.038. PubMed PMID:25199763 .
- Koncz, G, Hancz, A, Chakrabandhu, K, Gogolák, P, Kerekes, K, Rajnavölgyi, E et al.. Vesicles released by activated T cells induce both Fas-mediated RIP-dependent apoptotic and Fas-independent nonapoptotic cell deaths. J Immunol. 2012;189 (6):2815-23. doi: 10.4049/jimmunol.1102827. PubMed PMID:22891283 .
- Koncz, G, Hueber, AO. The Fas/CD95 Receptor Regulates the Death of Autoreactive B Cells and the Selection of Antigen-Specific B Cells. Front Immunol. 2012;3 :207. doi: 10.3389/fimmu.2012.00207. PubMed PMID:22848207 PubMed Central PMC3404404.
- Legros, L, Ebran, N, Stebe, E, Rousselot, P, Rea, D, Cassuto, JP et al.. Imatinib sensitizes T-cell lymphocytes from chronic myeloid leukemia patients to FasL-induced cell death: a brief communication. J Immunother. 2012;35 (2):154-8. doi: 10.1097/CJI.0b013e318243f238. PubMed PMID:22306903 .
- Legros, L, Slama, B, Karsenti, JM, Vey, N, Natarajan-Amé, S, Watel, E et al.. Treatment of myelodysplastic syndromes with excess of blasts by bevacizumab is well tolerated and is associated with a decrease of VEGF plasma level. Ann Hematol. 2012;91 (1):39-46. doi: 10.1007/s00277-011-1242-z. PubMed PMID:21553011 .
2009-2011 - Equipe Labellisée, La Ligue Contre le Cancer
2006-2011 - Contrat Hospitalier de Recherche Translationnelle (CHRT), Inserm-CHU
2006-2008 - Equipe Labellisée, La Ligue Contre le Cancer
2002 - Cancerology Price Raymond Rosen
2001 - ATIP CNRS
iBV - Institut de Biologie Valrose
"Sciences Naturelles"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2
