Nature Communications 2022 November 18. doi: 10.1038/s41467-022-34516-x
Biological effects of the loss of homochirality in a multicellular organism
Agnes Banreti1, Shayon Bhattacharya2, Frank Wien3, Koichi Matsuo4, Matthieu Réfrégiers5, Cornelia Meinert6, Uwe Meierhenrich6, Bruno Hudry1, Damien Thompson2, Stéphane Noselli1
Affiliations
1Université Côte d’Azur, CNRS, Inserm, Institut de Biologie Valrose; 06108, Nice, France.
2Department of Physics, Bernal Institute, University of Limerick; V94 T9PX, Limerick, Ireland.
3DISCO Beamline, Synchrotron SOLEIL; 91192, Gif-sur-Yvette, France.
4HiSOR Hiroshima Synchrotron Radiation Center, Hiroshima University; Hiroshima, Japan.
5Centre de Biophysique Moléculaire, CNRS; UPR4301, 45071 Orléans, France.
6Université Côte d’Azur, Institut de Chimie de Nice, CNRS; UMR 7272, 06108 Nice, France.
Abstract
Homochirality is an overarching design principle in all living organisms. RNA and DNA are exclusively built from D-sugars and protein biopolymers from L-amino acids, with any departure from this primary condition being detrimental to life.
Recent advances demonstrate that self-proteins can undergo spontaneous chiral posttranslational modifications under conditions of cell stress and aging. While these indicate that the maintenance of homochirality is central to biology, the mechanisms by which the adverse accumulation of non-L-amino-acids in proteins lead to pathophysiological consequences remain poorly understood. This was due to the absence of specific tools and model system to study dynamic chirality changes in vivo.
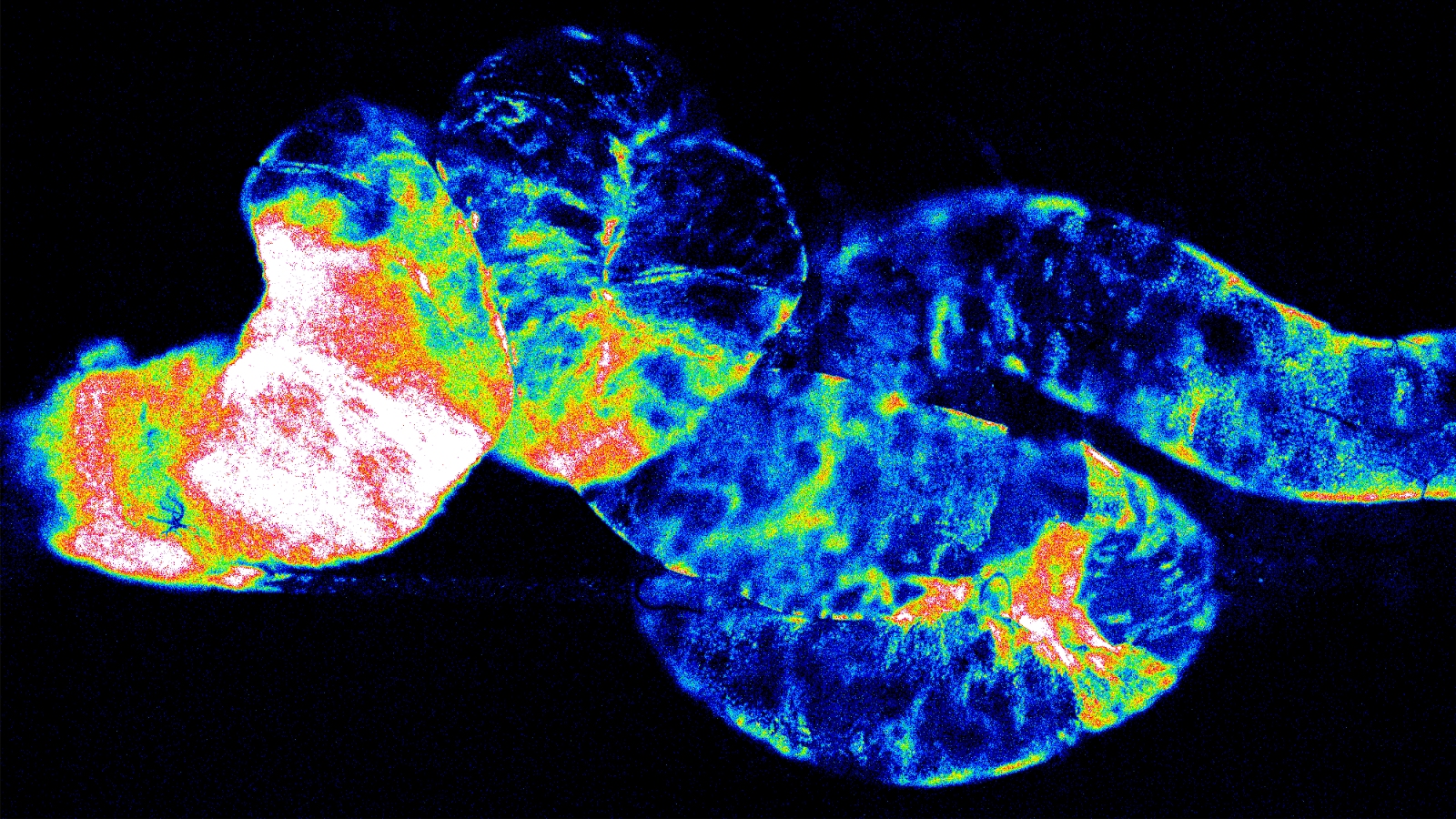
To address how heterochirality build-up impacts organism’s health, we developed interdisciplinary chiral-selective techniques to detect protein-bound non-l-amino acids and assessed their functional significance in Drosophila.
Our results show that accumulation of non-L-AAs in proteins, promotes a progressive heterochirality syndrome, through a cascading effect across biological scales spanning from loss of molecular homochirality to increased resistance to caspase activity in cells, increased tumour susceptibility in organs and, consequently, premature death of the chiral-deficient animal.
We further suggest that heterochirality spreading in living organisms represents a novel causal factor that may be associated with a broad range of defective cellular processes, diseases and ageing. Developing methods for monitoring heterochirality in tissues, such as those described in this work, will provide new avenues for both basic and biomedical research and the study of cell signalling in altered chiral environments.
DOI: 10.1038/s41467-022-34516-x

