
Alberto ROSELLO-DIEZ
Adaptive Regulation of Organ Size (AROS)
Main interests
- Identify the mechanisms that regulate the number and behaviours of cartilage progenitors during limb development and repair
- Determine the role of mechanotransduction and bioelectricity in the control of appendage size during development and regeneration
- Study the control of limb:body proportions limb-specific inter-species chimeras in rodents
- Develop synthepods (limb assembloids) with cells of multiple species to study the control of developmental tempo
Scientific Questions
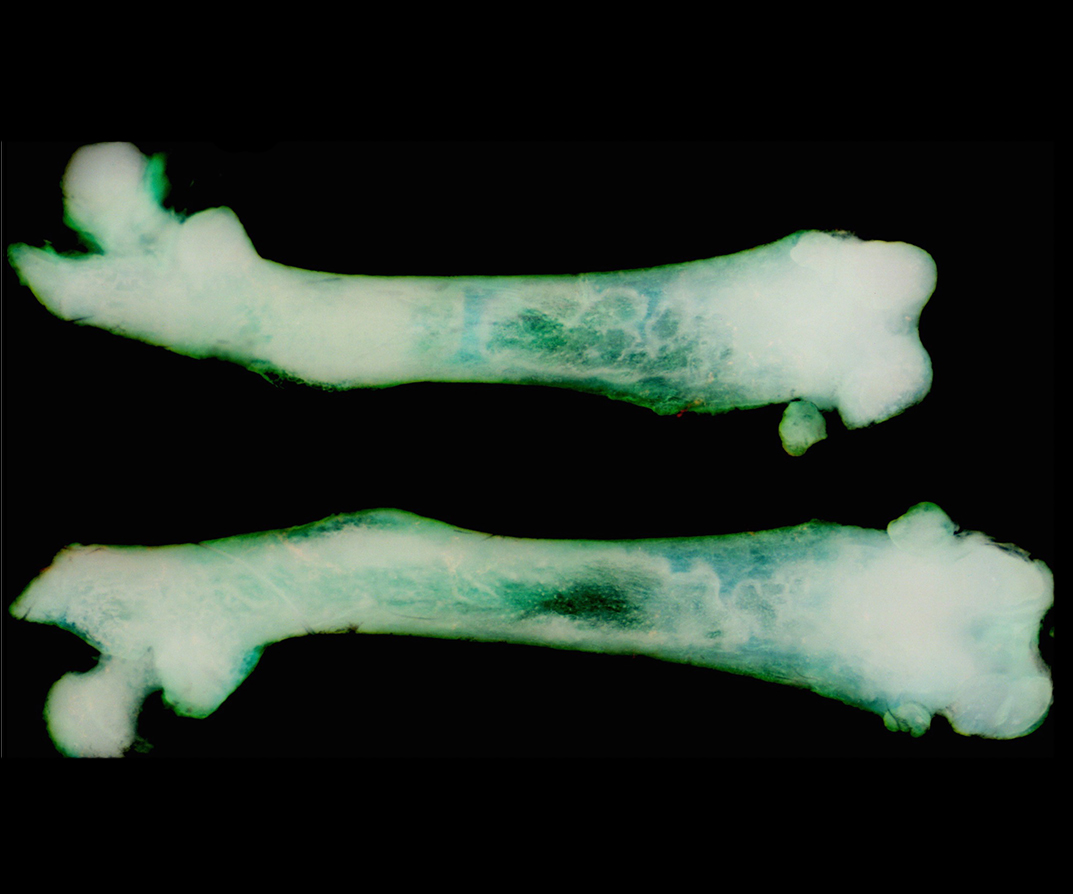
The control of organ size during development and regeneration is one of the last frontiers of biology, as we lack a fundamental understanding of how organ size is encoded and sensed. A related question is how growth is coordinated with developmental tempo (the timing and speed at which embryos go over developmental stages). Specifically, we are interested in the following questions:
How are the number and behaviours of stem/progenitor cells controlled in response to growth status?
How is final organ size established in development and regeneration?
How are body proportions established and maintained?
Our Strategy
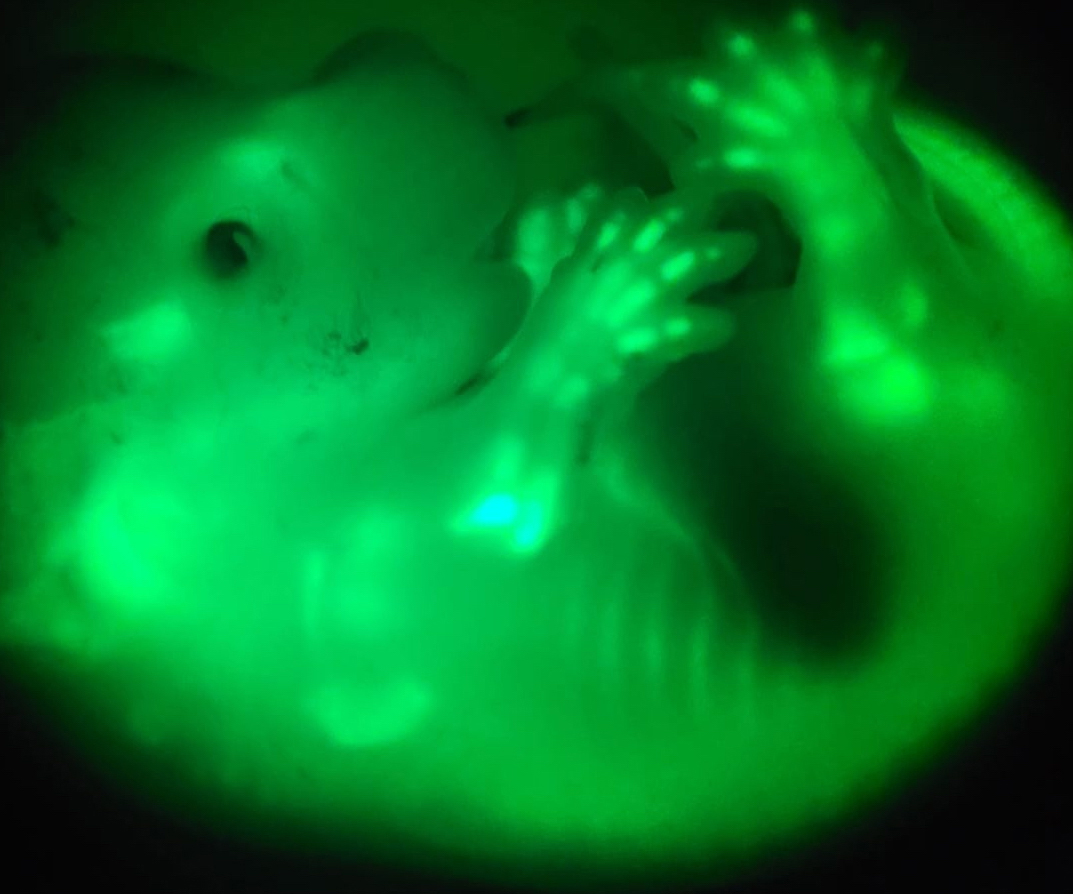
We use limb development and regeneration to study these questions, with a focus on the long bones. Sophisticated mouse genetics and rodent and avian embryo micromanipulation are used to challenge the system and study how it adapts. Two types of experiments are used: a) Recovery after transient growth perturbation and b) inter-species chimeras, in which limbs made of cells of other species develop in a mouse body. For analysis, we use multi-colour lineage tracing, spatio-temporal characterisation of proliferative and differentiation potential, and bulk and single-cell multi-omics (and spatial transcriptomics in the near future) to reveal the local mechanisms that restore tissue size and integrity, and the systemic ones that establish and maintain body proportions. In future years, we also plan to use zebrafish to study the role of bioelectricity in the control of fin size during regeneration.
Research Aims
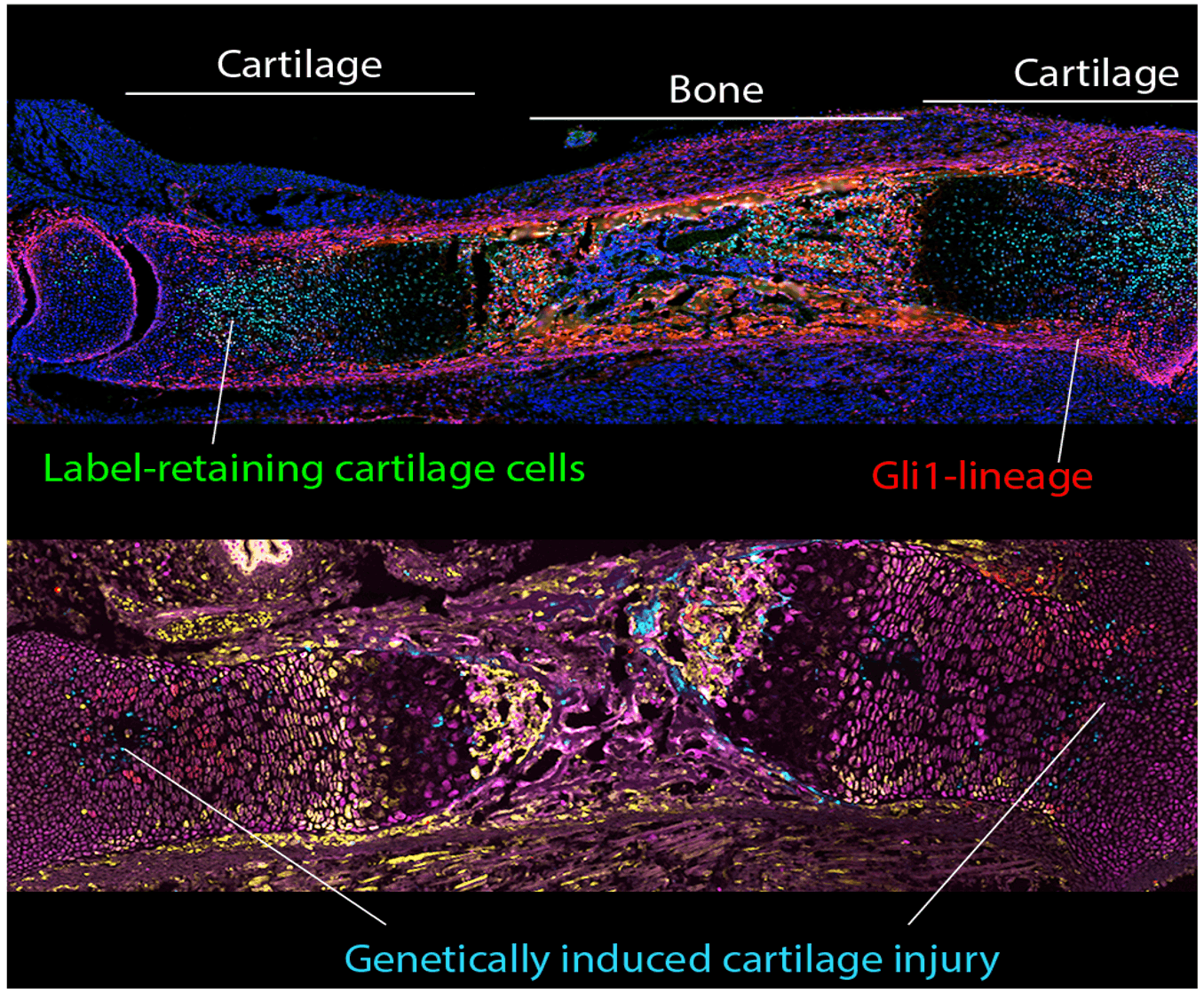
Unravelling the regulation of Gli1+ cartilage progenitors in response to growth status
Long bones grow via a transient cartilage template that is progressively replaced by bone. The number and behaviour of cartilage progenitors has to be tightly regulated, such that cartilage destruction and production are perfectly balanced to sustain bone growth. We have recently described Gli1+ cells as the fetal precursors of long-lived cartilage progenitors, and we are currently exploring how they are regulated. We will artificially manipulate their numbers in vivo (Figure) to study if/how they go back to normal, and these manipulations will be combined with genetic modulation of candidate signalling pathways that we hypothesise link growth status with Gli1+ cell numbers.
Figure. Fetal mouse tibias showcasing genetic models for manipulation and tracing of target cell populations
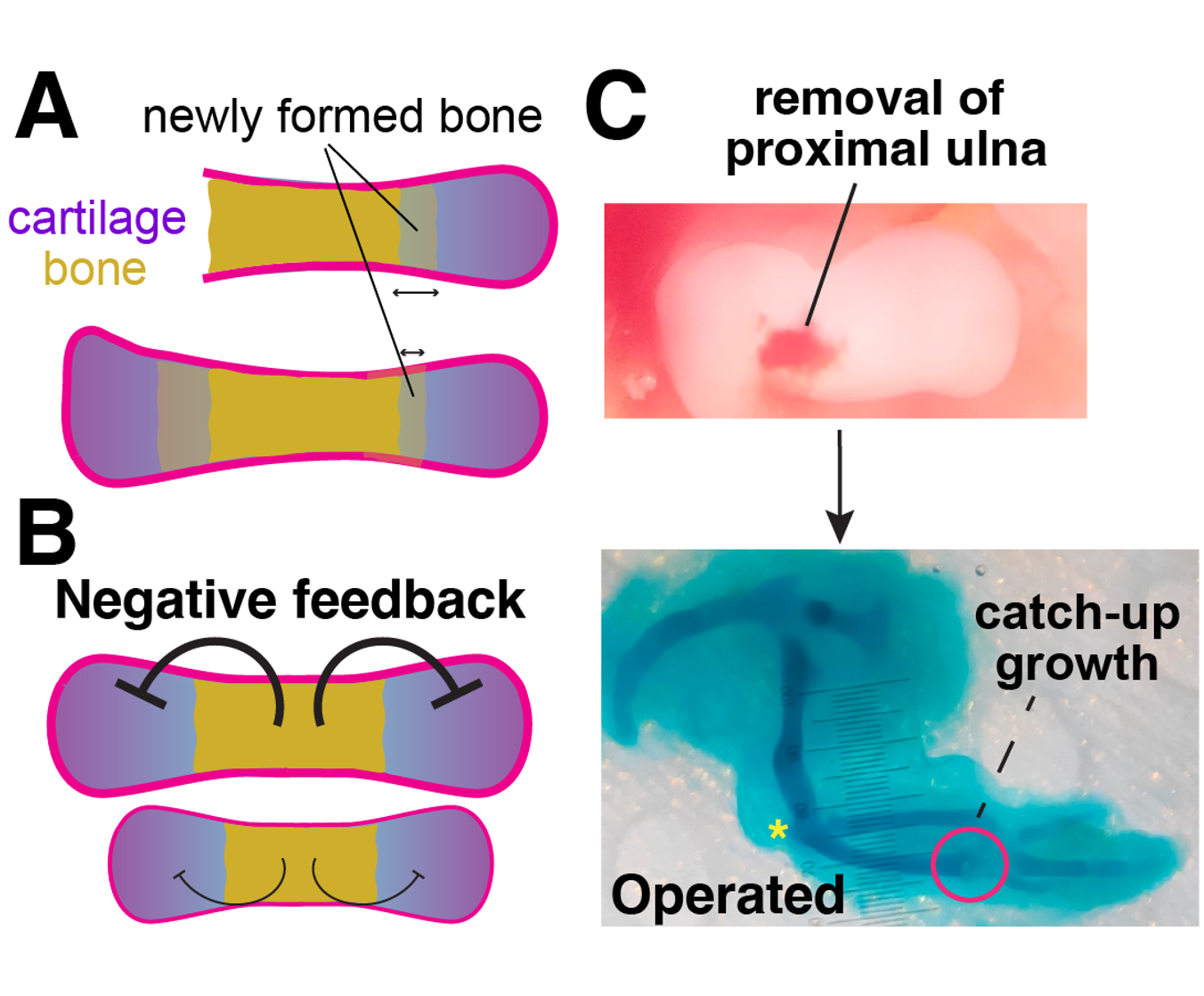
Exploring the control of final bone length by mechanotransduction
What stops bone growth when a species-specific length is achieved? In other words, does the cartilage adapt its activity based on bone length? Classic experiments showed that, when one of the cartilage poles is destroyed, the remaining one produces more bone than normal (Figure panel A). This suggests a model in which negative feedback is generated in response to bone elongation, increasingly inhibiting cartilage activity (panel B). We have developed a surgical model in chicken embryos whereby removal of the proximal ulna cartilage leads to overgrowth from the distal ulna (panel C). We will use tension reporters and spatial transcriptomics to study whether changes in the mechanical environment due to bone elongation are converted into bone-length information, interpreted by the cartilage.
Figure. A, Unipolar cartilage destruction triggers overgrowth of the spared cartilage. B, proposed negative feedback model. C, new chicken model to study the negative-feedback control of cartilage activity
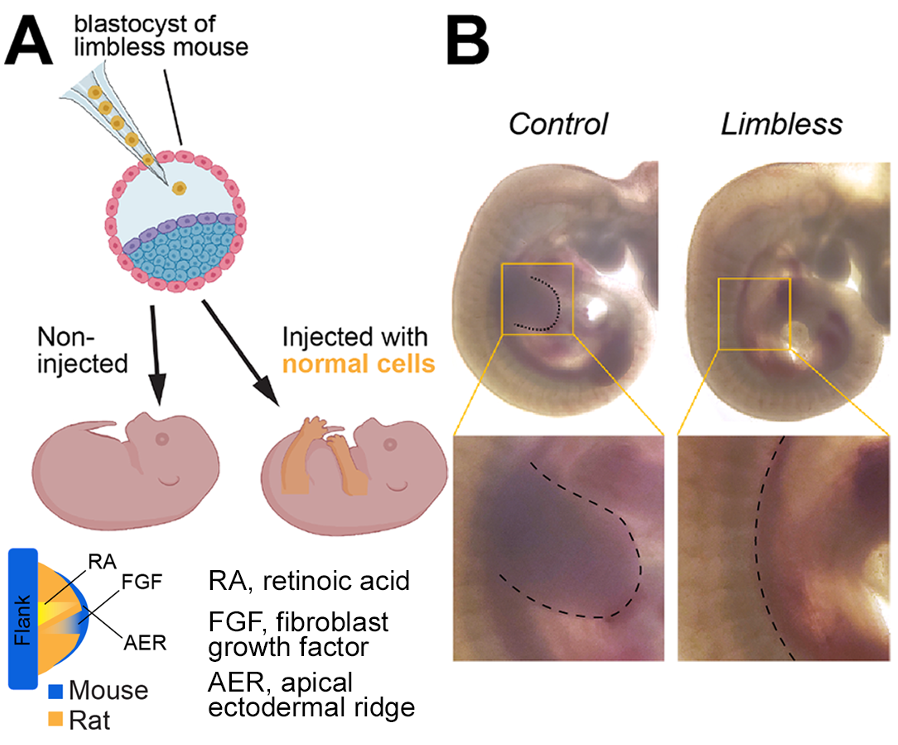
Using limb-enriched interspecies chimeras to study the control of limb:body proportions
A classic approach to study limb-size determination is to cross-graft limb primordia (a.k.a. buds) between close species that differ in size. Initial experiments suggested that the size of the donor limb was unaffected or only slightly modulated by the host (Twitty 1931). However, we showed that fate plasticity declines with developmental age. Hence, by the time a limb bud is big enough to be grafted, it might already be impervious to reprogramming by extrinsic signals. To circumvent this caveat, we are generating for the first time chimeric animals in which stem cells from a different species are injected into early mouse embryos unable to form the limbs (see Figure), so that the injected cells give rise to the limb tissues in the context of the host’s signals. We have established international collaborations to obtain stem cells from multiple species, characterised by different body sizes, limb:body proportions and developmental tempos.
Figure. A, Blastocyst complementation to generate limb-enriched inter-species chimeras. B, Limbless model.
Recent publications
- Rosello-Diez, A, Menchero, S. In preprints: oxygen and NFκB signals shift the timing of hindlimb formation. Development. 2024;151 (24):. doi: 10.1242/dev.204578. PubMed PMID:39705658 .
- Zhou, W, Van Sinderen, M, Rainczuk, K, Menkhorst, E, Sorby, K, Osianlis, T et al.. Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion. Proc Natl Acad Sci U S A. 2024;121 (41):e2401071121. doi: 10.1073/pnas.2401071121. PubMed PMID:39365817 PubMed Central PMC11474043.
- Ho H'ng, C, Amarasinghe, SL, Zhang, B, Chang, H, Qu, X, Powell, DR et al.. Compensatory growth and recovery of cartilage cytoarchitecture after transient cell death in fetal mouse limbs. Nat Commun. 2024;15 (1):2940. doi: 10.1038/s41467-024-47311-7. PubMed PMID:38580631 PubMed Central PMC10997652.
- H'ng, CH, Khaladkar, A, Rosello-Diez, A. Look who's TORking: mTOR-mediated integration of cell status and external signals during limb development and endochondral bone growth. Front Cell Dev Biol. 2023;11 :1153473. doi: 10.3389/fcell.2023.1153473. PubMed PMID:37152288 PubMed Central PMC10154674.
- McCusker, C, Rosello-Diez, A. In preprints: new insights into proximodistal limb patterning and differentiation. Development. 2022;149 (19):. doi: 10.1242/dev.201308. PubMed PMID:36200555 PubMed Central PMC10655916.
- Beltran Diaz, S, H'ng, CH, Qu, X, Doube, M, Nguyen, JT, de Veer, M et al.. A New Pipeline to Automatically Segment and Semi-Automatically Measure Bone Length on 3D Models Obtained by Computed Tomography. Front Cell Dev Biol. 2021;9 :736574. doi: 10.3389/fcell.2021.736574. PubMed PMID:34513850 PubMed Central PMC8427701.
- Bandyopadhyay, A, Francis-West, P, Katti, D, Roselló-Díez, A. Musculoskeletal development, maintenance and regeneration: Part two. Dev Dyn. 2021;250 (3):300-301. doi: 10.1002/dvdy.314. PubMed PMID:33580530 .
- Bandyopadhyay, A, Francis-West, P, Katti, D, Roselló-Díez, A. Musculoskeletal Development, Maintenance and Regeneration: Part One. Dev Dyn. 2021;250 (1):6-7. doi: 10.1002/dvdy.277. PubMed PMID:33295101 .
- Rosello-Diez, A. Size and scale during development and regeneration. Wiley Interdiscip Rev Dev Biol. 2020;9 (6):e393. doi: 10.1002/wdev.393. PubMed PMID:32786055 .
- Delgado, I, López-Delgado, AC, Roselló-Díez, A, Giovinazzo, G, Cadenas, V, Fernández-de-Manuel, L et al.. Proximo-distal positional information encoded by an Fgf-regulated gradient of homeodomain transcription factors in the vertebrate limb. Sci Adv. 2020;6 (23):eaaz0742. doi: 10.1126/sciadv.aaz0742. PubMed PMID:32537491 PubMed Central PMC7269661.
- Kagan, BJ, Rosello-Diez, A. Integrating levels of bone growth control: From stem cells to body proportions. Wiley Interdiscip Rev Dev Biol. 2021;10 (1):e384. doi: 10.1002/wdev.384. PubMed PMID:32436370 .
- Ahmadzadeh, E, Bayin, NS, Qu, X, Singh, A, Madisen, L, Stephen, D et al.. A collection of genetic mouse lines and related tools for inducible and reversible intersectional mis-expression. Development. 2020;147 (10):. doi: 10.1242/dev.186650. PubMed PMID:32366677 PubMed Central PMC7272339.
- Willett, RT, Bayin, NS, Lee, AS, Krishnamurthy, A, Wojcinski, A, Lao, Z et al.. Cerebellar nuclei excitatory neurons regulate developmental scaling of presynaptic Purkinje cell number and organ growth. Elife. 2019;8 :. doi: 10.7554/eLife.50617. PubMed PMID:31742552 PubMed Central PMC6890462.
- Rosello-Diez, A, Whited, JL. Discussing limb development and regeneration in Barcelona: The future is at hand. Dev Dyn. 2020;249 (2):160-163. doi: 10.1002/dvdy.121. PubMed PMID:31587395 .
- Uribe, V, Rosello-Diez, A. Culturing and Measuring Fetal and Newborn Murine Long Bones. J Vis Exp. 2019; (146):. doi: 10.3791/59509. PubMed PMID:31081827 .
- Roselló-Díez, A, Madisen, L, Bastide, S, Zeng, H, Joyner, AL. Cell-nonautonomous local and systemic responses to cell arrest enable long-bone catch-up growth in developing mice. PLoS Biol. 2018;16 (6):e2005086. doi: 10.1371/journal.pbio.2005086. PubMed PMID:29944650 PubMed Central PMC6019387.
- Roselló-Díez, A, Stephen, D, Joyner, AL. Altered paracrine signaling from the injured knee joint impairs postnatal long bone growth. Elife. 2017;6 :. doi: 10.7554/eLife.27210. PubMed PMID:28741471 PubMed Central PMC5526667.
- Legué, E, Gottshall, JL, Jaumouillé, E, Roselló-Díez, A, Shi, W, Barraza, LH et al.. Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern. Neural Dev. 2016;11 (1):17. doi: 10.1186/s13064-016-0072-z. PubMed PMID:27609139 PubMed Central PMC5017010.
- Roselló-Díez, A, Joyner, AL. Regulation of Long Bone Growth in Vertebrates; It Is Time to Catch Up. Endocr Rev. 2015;36 (6):646-80. doi: 10.1210/er.2015-1048. PubMed PMID:26485225 PubMed Central PMC4702496.
- Zheng, HF, Forgetta, V, Hsu, YH, Estrada, K, Rosello-Diez, A, Leo, PJ et al.. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526 (7571):112-7. doi: 10.1038/nature14878. PubMed PMID:26367794 PubMed Central PMC4755714.
2020 - John Haddad Young Investigator Award. American Society for Bone and Mineral Research.
2019 - Career Development Award. Human Frontiers Science Program.
2015 - Charles Revson Senior Fellowship in Biomedical Science.
2012 - HFSP Long-Term Postdoctoral Fellowship. Human Frontiers Science Program.
2011 - EMBO Long-Term Postdoctoral Fellowship. EMBO. Declined in favour of HFSP.
2011 - Doctorate Extraordinary Prize. Science Faculty of the Autonomous Univ. of Madrid, Spain.
2007 - Selected to attend the 57th Lindau Nobel Laureate Meeting (Physiology and Medicine).
2006 - First National Extraordinary Prize for best track record in Biochemistry in Spain.
iBV - Institut de Biologie Valrose
"Sciences Naturelles"
Université Nice Sophia Antipolis
Faculté des Sciences
Parc Valrose
06108 Nice cedex 2



