Nat Commun. 2024 Jan 2;15(1):65. doi: 10.1038/s41467-023-44548-6
Hijacking of internal calcium dynamics by intracellularly residing viral rhodopsins
Ana-Sofia Eria-Oliveira1,2,3,4, Mathilde Folacci1,3,5, Anne Amandine Chassot2,3,4, Sandrine Fedou6, Nadine Thézé6, Dmitrii Zabelskii7, Alexey Alekseev8,9, Ernst Bamberg10, Valentin Gordeliy1,11, Guillaume Sandoz12,13,14, Michel Vivaudou15,16
Affiliations
1Univ. Grenoble Alpes, CEA, CNRS, IBS, Grenoble, France.
2Université Côte d’Azur, CNRS, INSERM, iBV, Nice, France.
3Laboratories of Excellence, Ion Channel Science and Therapeutics, Nice, France.
4Fédération Hospitalo-Universitaire InovPain, Cote d’Azur University, University Hospital Center Nice, Nice, France.
5Department of Biomedicine, Aarhus University, Aarhus, Denmark.
6Univ. Bordeaux, Inserm, BRIC, UMR, 1312, Bordeaux, France.
7European XFEL, Schenefeld, Germany.
8Advanced Optogenes Group, Institute for Auditory Neuroscience and InnerEarLab, University Medical Center Göttingen, Göttingen, Germany.
9Cluster of Excellence “Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells” (MBExC), University of Göttingen, Göttingen, Germany.
10Max Planck Institute of Biophysics, Frankfurt am Main, Germany.
11Institute of Biological Information Processing (IBI-7: Structural Biochemistry), Forschungszentrum Jülich, Jülich, Germany.
12Université Côte d’Azur, CNRS, INSERM, iBV, Nice, France.
13Laboratories of Excellence, Ion Channel Science and Therapeutics, Nice, France.
14Fédération Hospitalo-Universitaire InovPain, Cote d’Azur University, University Hospital Center Nice, Nice, France.
15Univ. Grenoble Alpes, CEA, CNRS, IBS, Grenoble, France.
16Laboratories of Excellence, Ion Channel Science and Therapeutics, Nice, France.
Abstract
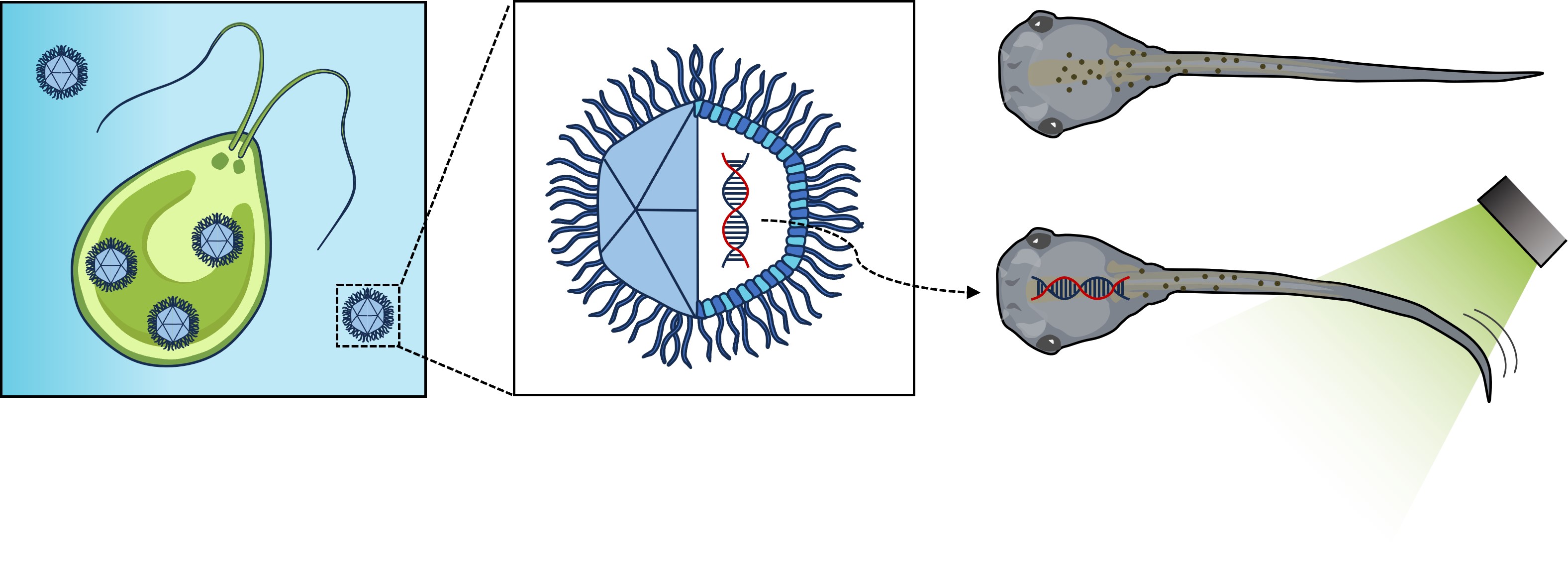
Rhodopsins are ubiquitous light-driven membrane proteins with diverse functions, including ion transport. Widely distributed, they are also coded in the genomes of giant viruses infecting phytoplankton where their function is not settled. Here, we examine the properties of OLPVR1 (Organic Lake Phycodnavirus Rhodopsin) and two other type 1 viral channelrhodopsins (VCR1s), and demonstrate that VCR1s accumulate exclusively intracellularly, and, upon illumination, induce calcium release from intracellular IP3-dependent stores. In vivo, this light-induced calcium release is sufficient to remote control muscle contraction in VCR1-expressing tadpoles. VCR1s natively confer light-induced Ca2+ release, suggesting a distinct mechanism for reshaping the response to light of virus-infected algae. The ability of VCR1s to photorelease calcium without altering plasma membrane electrical properties marks them as potential precursors for optogenetics tools, with potential applications in basic research and medicine.
DOI: 10.1038/s41467-023-44548-6

