Matrix Biol. 2022 Dec. doi: 10.1016/j.matbio.2022.10.004
Regulation of the collagen IV network by the basement membrane protein perlecan is crucial for squamous epithelial cell morphogenesis and organ architecture
Raphaël Bonche1, Prune Smolen1, Aline Chessel1, Séverine Boisivon1, Sabrina Pisano2, Aaron Voigt3, Sébastien Schaub1, Pascal Thérond1, Sandrine Pizette4
Affiliations
1Université Côte d’Azur, CNRS, Inserm, iBV, France.
2Université Côte d’Azur, CNRS, Inserm, IRCAN, France.
3Department of Neurology, University Medical Center, RWTH Aachen University, Aachen 52074, Germany.
4Université Côte d’Azur, CNRS, Inserm, iBV, France.
Abstract
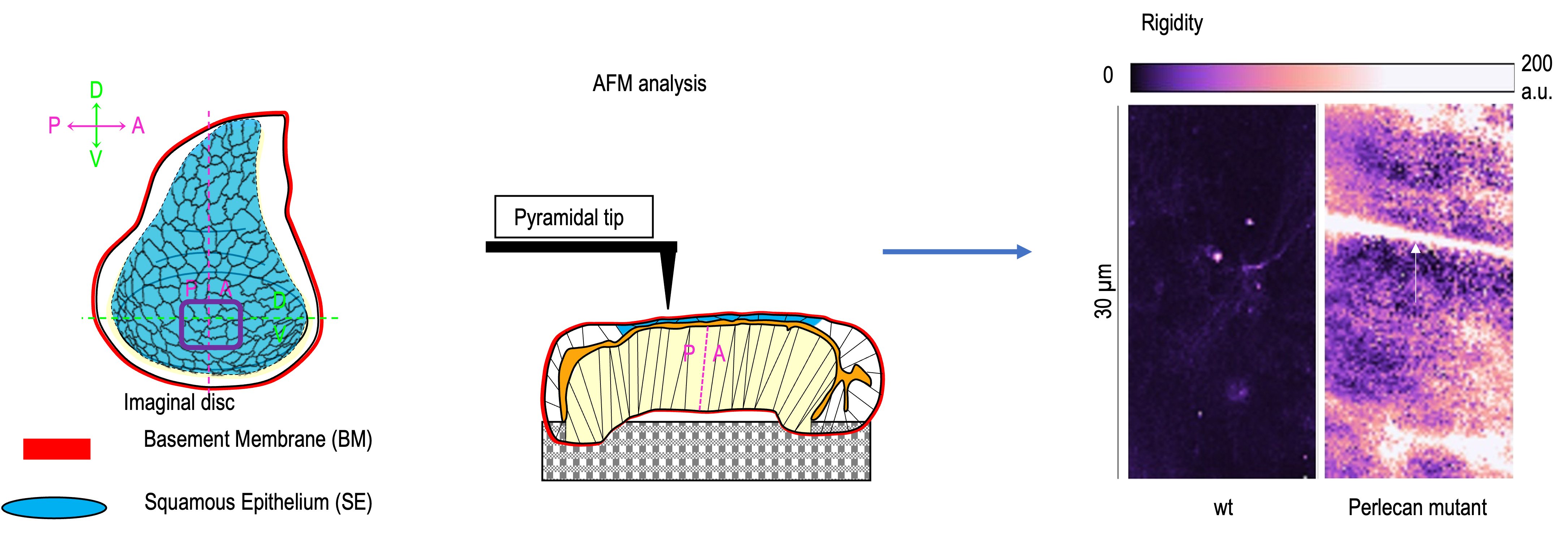
All epithelia have their basal side in contact with a specialized extracellular matrix, the basement membrane (BM). During development, the BM contributes to the shaping of epithelial organs via its mechanical properties. These properties rely on two core components of the BM, collagen type IV and perlecan/HSPG2, which both interact with another core component, laminin, the initiator of BM assembly. While collagen type IV supplies the BM with rigidity to constrain the tissue, perlecan antagonizes this effect. Nevertheless, the number of organs that has been studied is still scarce, and given that epithelial tissues exhibit a wide array of shapes, their forms are bound to be regulated by distinct mechanisms. This is underscored by mounting evidence that BM composition and assembly/biogenesis is tissue-specific. Moreover, previous reports have essentially focused on the mechanical role of the BM in morphogenesis at the tissue scale, but not the cell scale. Here, we took advantage of the robust conservation of core BM proteins and the limited genetic redundancy of the Drosophila model system to address how this matrix shapes the wing imaginal disc, a complex organ comprising a squamous, a cuboidal and a columnar epithelium. With the use of a hypomorphic allele, we show that the depletion of Trol (Drosophila perlecan) affects the morphogenesis of the three epithelia, but particularly that of the squamous one. The planar surface of the squamous epithelium (SE) becomes extremely narrow, due to a function for Trol in the control of the squamous shape of its cells. Furthermore, we find that the lack of Trol impairs the biogenesis of the BM of the SE by modifying the structure of the collagen type IV lattice. Through atomic force microscopy and laser surgery, we demonstrate that Trol provides elasticity to the SE’s BM, thereby regulating the mechanical properties of the SE. Moreover, we show that Trol acts via collagen type IV, since the global reduction in the trol mutant context of collagen type IV or the enzyme that cross-links its 7S -but not the enzyme that cross-links its NC1- domain substantially restores the morphogenesis of the SE. In addition, a stronger decrease in collagen type IV achieved by the overexpression of the matrix metalloprotease 2 exclusively in the BM of the SE, significantly rescues the organization of the two other epithelia. Our data thus sustain a model in which Trol counters the rigidity conveyed by collagen type IV to the BM of the SE, via the regulation of the NC1-dependant assembly of its scaffold, allowing the spreading of the squamous cells, spreading which is compulsory for the architecture of the whole organ.
DOI: 10.1016/j.matbio.2022.10.004

