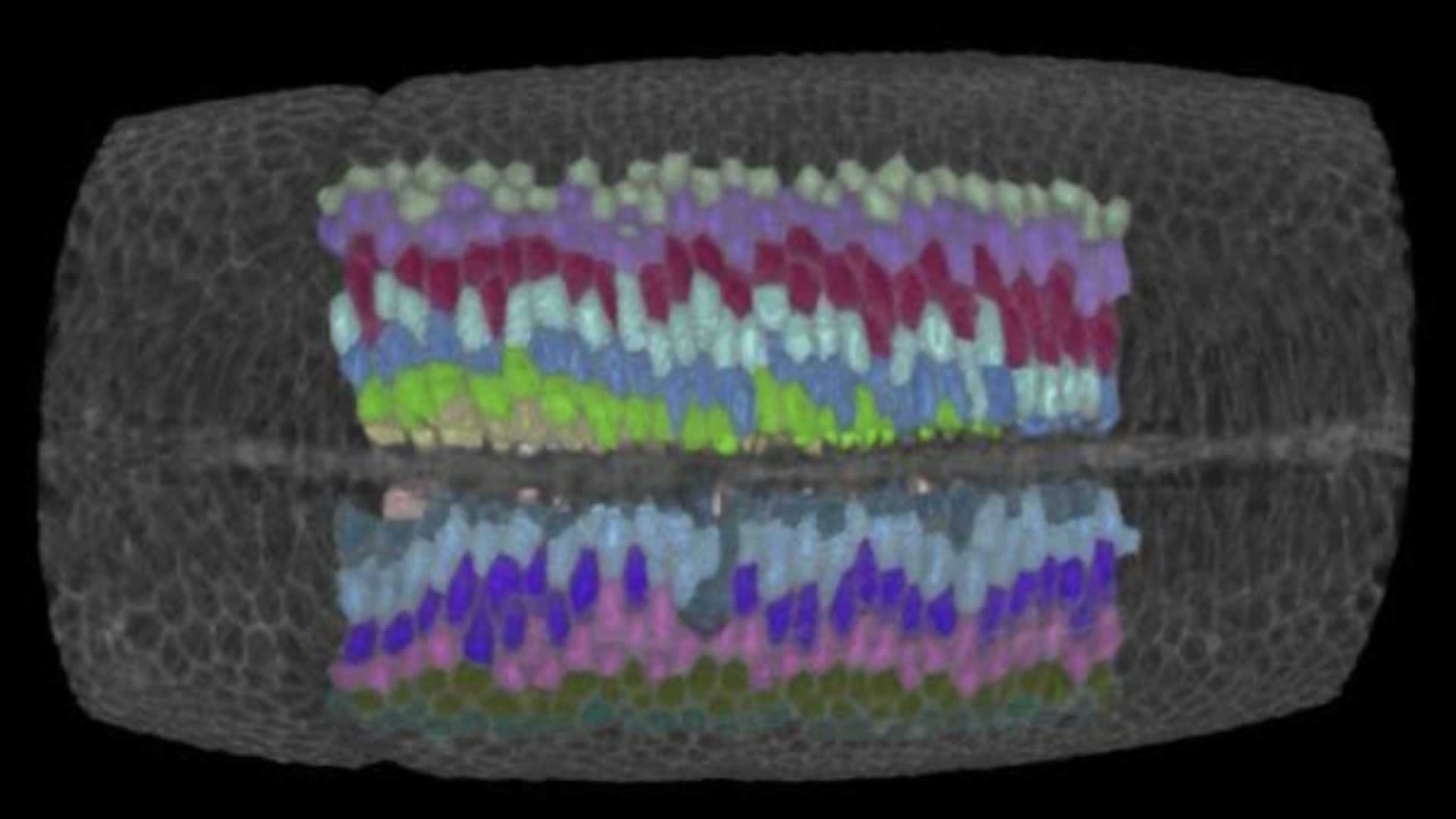
Embryo gastrulation is the process by which the blastoderm tissue is remodeled and different groups of cells are translocated in different parts of the embryo to eventually form the right organ at the right place and time. In Drosophila, gastrulation is initiated by the formation of a furrow along the ventral side of the embryo blastoderm (the future mesoderm tissue). Unravelling the mechanism driving ventral furrow formation is therefore primordial to understand how the embryo initiates the morphogenetic changes that will eventually result in a living animal.
Ventral furrow formation has been proposed to result from the collective wedging of prospective mesoderm cells: the cells located at the ventral side of the embryo decrease their apical while enlarge their basal surface area forming a trapezoidal shape. This concerted change in cell shape has been hypothesized to result from apical-basal differential tension that induces a torque driving a bending moment of the epithelium.
The study by the Rauzi lab in collaboration with other two teams at the University of Grenoble and Cambridge University has now challenged this long-lasting mechanical model: this work shows that cell wedging is not necessary to form the ventral furrow. This is supported by the evidence, for instance, that mutant embryos that do not form cells (i.e., that are torque free) still form a furrow. By implementing 3D computational modelling, multi-view light sheet microscopy, multidimensional morphometric analysis and laser based tissue manipulation, this study shows that the embryo establishes a contractile string along the ventral midline working like a ‘cheese cutter wire’ that indents the ventral side of the embryo forming a furrow. The ventral contractile string results from an actomyosin supracellular meshwork that pulls on the embryo polar regions resisting the contractile stress and therefore acting as anchoring sites. Under these conditions, the furrow propagates bi-directionally from the central to anterior/posterior polar regions of the embryo. Furrow propagation is a process that has been often reported during epithelial tube formation undergoing wrapping (e.g., during neural tube formation). Propagation of a fold can be triggered by the propagation of a signaling or mechanical wave along a determined path. Here, this study shows that, even in absence of triggering waves, the process of furrow formation and propagation can emerge from the long-range mechanical interaction of epithelial tissues.
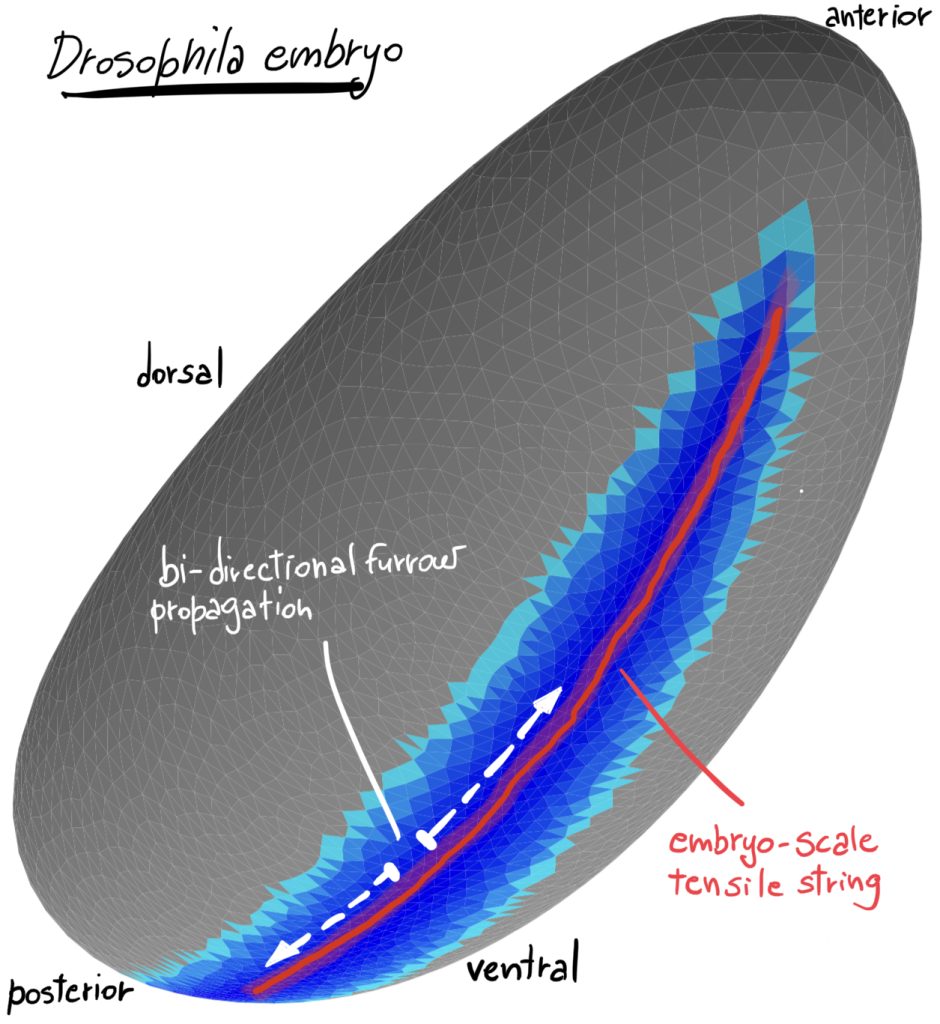
Legend. 3D rendering of an in silico simulation of the Drosophila embryo at the onset of gastrulation. Darker blue indicates greater apical surface constriction. The red line indicates the region of greatest tensile stress working as a ‘cheese cutter wire’ indenting the ventral tissue and initiating embryo gastrulation.
This work was published in Nature Communications and highlighted by INSB (Institut des Sciences Biologiques du CNRS).
For more information
“Embryo-scale epithelial buckling forms a propagating furrow that initiates gastrulation”
Julien FIERLING, Alphy JOHN, Barthélémy DELORME, Alexandre TORZYNSKI, Guy B. BLANCHARD, Claire M. LYE, Anna POPKOVA, Grégoire MALANDAIN, Bénédicte SANSON, Jocelyn ÉTIENNE, Philippe MARMOTTANT, Catherine QUILLIET, and Matteo RAUZI – Nature Communications – DOI doi.org/10.1038%2Fs41467-022-30493-3