Understanding the relationships between cancer cells and the tumor ecosystem is a major challenge to understand the pathophysiology and identify new therapeutic approaches in pancreatic adenocarcinoma (AdKP), one of the most aggressive solid cancers, with a 5-year survival rate of less than 10%.
In AdKP, the tumor microenvironment is mainly composed of immune cells, nerve fibers, vessels and cancer associated fibroblasts (CAFs). CAFs are key elements of the stroma and are derived from fibroblasts, the support cells naturally present in the pancreas. CAFs play a major role not only in disease progression, but also in treatment resistance. Under the influence of tumor cells, these fibroblasts proliferate, secrete chemical signals and proteins that form a network, the extracellular matrix. This very particular environment controls the tumor cells in return by stimulating their pro-invasive functions. More aggressive cancer cells form metastases that colonize distant organs, in particular the liver. The lack of knowledge about the molecular basis of communication between CAFs and cancer cells prevents the development of effective therapeutic options targeting this intercellular interaction. Understanding the communication pathways between tumor cells and CAFs represents a major challenge for the emergence of new treatments.
Olivier Soriani’s team researchers have focused on proteins that are still little studied in cancer research, the ion channel families. In this study, they reveal how SK2, a potassium channel, stimulates the formation of metastases by sensitizing cancer cells to signals from the tumor microenvironment. Ion channels are membrane nanosensors that translate perturbations in the microenvironment into electrical signals that can be interpreted by cells. Researchers evaluated the paracrine effects of human tumor-derived CAFs on the electrical characteristics of pancreatic cancer cells (PCCs).
The molecular mechanisms were decrypted through a combination of electrophysiology, bioinformatics, molecular biology and biochemistry techniques in cell lines and human samples.
An orthotopic mouse model, in which CAF and PCC were co-injected, was used to evaluate tumor growth and metastasis dissemination. Pharmacological studies were performed in a spontaneous AdKP generating mouse model (KICpdx1).
The team established that:
1-CAF-induced stimulations of cancer cell invasive properties require the activation of the SK2 channels.
2-SK2 activity is increased by direct AKT-mediated phosphorylation of the channel which in turn sensitizes a β1-integrin–EGFR–AKT signaling hub.
3-Targeting SigmaR1, a chaperone of SK2, shuts the signaling hub off, inhibits liver metastasis formation and increases in survival in PDAC mouse models.
This work demonstrates for the first time the role of ion channels in the dialogue between tumor cells and the actors of their ecosystem. These findings highlight the SK2 channel as an original target to counteract stromal-induced cancer cell aggressiveness in PDAC that can be targeted through Sig-1R, a druggable chaperone.
Further studies will clarify the role of this new therapeutic pathway using sigma ligands as an adjuvant to reference treatments or as a first line treatment. The prospects offered by these results could be extended to other cancers in which the role of the stroma is predominant (breast or colon cancer).
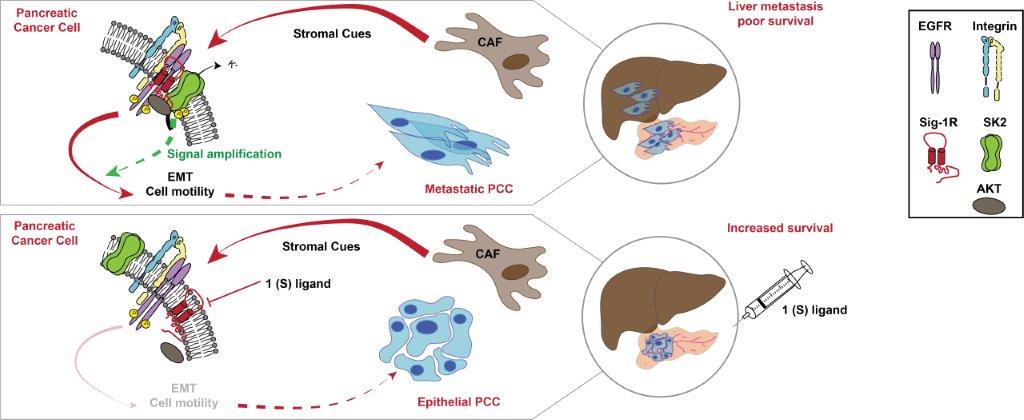
Legend. Schematic diagram showing the mechanism by which CAF secretome activates an SK2-dependent β1-integrin–EGFR–AKT signalling hub that promotes PCC aggressiveness. This mechanism can be pharmacologically targeted through Sig1-R to counteract the intercellular communication between CAF and PCC in PDAC (CAF, cancer-associated fibroblasts; EMT, epithelial-to-mesenchymal transition; PCC, pancreatic cancer cells; PDAC, pancreatic ductal adenocarcinoma).
This work has been published in the journal GUT and highlighted by Université Côte d’Azur
For more information
“SK2 channels set a signalling hub bolstering CAF-triggered tumourigenic processes in pancreatic cancer”
Raphael Rapetti-Mauss , Jérémy Nigri , Camille Berenguier, Pascal Finetti, Sarah Simha Tubiana, Bonnie Labrum, Benoit Allegrini, Bernard Pellissier, Georgios Efthymiou, Zainab Hussain , Corinne Bousquet , Nelson Dusetti , François Bertucci, Hélène Guizouarn, Patricia Melnyk, Franck Borgese, Richard Tomasini , Olivier Soriani
Gut 2022;0:1–14. doi:10.1136/gutjnl-2021-326610