Improving cancer treatment requires a better understanding of how cancer stem cells (CSC) are maintained in solid tumors. By studying samples of human glioblastoma, a particularly aggressive brain tumor, the Thierry Virolle team has shown an enrichment of this aggressive cell population by the conversion of differentiated tumor cells into CSC. This study, associating clinicians from Nice University Hospital and researchers from iBV, revealed new molecular targets for targeted therapies. This work was published in the journal Cancer research.
Glioblastomas (GBM) are the most common form of primary brain tumors afflicting adult patients of all ages. These high-grade vascularized and infiltrating tumors remain incurable and lead to a fatal outcome in less than 18 months. Only a small proportion of patients live for more than 3 years, and very few unfortunately beyond 5 years after surgery. This failure of conventional therapies can be explained by the selection and amplification of a subpopulation of aggressive and very resistant tumor cells, glioma stem cells (GSC).
GSCs are a subpopulation of aggressive tumor cells, with stem cell properties (self-renewal, differentiation) and drug resistance. As a consequence, GSCs are responsible for the initiation and progression of tumors as well as recurrences, unfortunately systematic for this pathology. Targeting of GSCs is therefore a prerequisite for improving the therapeutic management of glioblastomas.
In this context, the studies developed by the team of Thierry Virolle intend to better understand the molecular mechanisms involved in the maintenance of the aggressive properties of GSCs or their engagement in differentiated tumor cells of low malignancy. GSC and tumor differentiated cells coexist in the tumor microenvironment, either intermingled or segregated as functionally divergent tumor territories and contribute to tumor maintenance, growth and propagation in function of their differentiation status and their ability to resist to conventional treatments: radiotherapy and chemotherapy.
The team brings together research staff (Laurent Turchi, engineer and Thierry Virolle, director of research Inserm) and clinicians (Fabien Almairac, neurosurgeon, and Fanny Burel Vandenbos, anatomopathologist), an essential association to link fundamental research with clinical applications (see inset).
Since 2009, they have established with two Nice university-hospital services, the Neurosurgery Department and the Central Pathological Anatomy Laboratory, a pipeline allowing the supply of characterized primary brain tumors. This collaboration allowed to generate a series of GSC primary cultures as well as an original collection of tumor samples. These samples are representative of different tumor territories containing both non aggressive differentiated cells as well as very aggressive GSC. By examining these samples, researchers discovered that differentiated tumor cells were capable, under certain conditions, of acquiring all the properties of GSC and that this conversion is strictly controlled by a “molecular toggle switch” (see inset) which allows to trigger the transition. Moreover, they highlighted that this switch can work both ways: If the differentiated tumor cells can transform into very aggressive cancer stem cells, the opposite path is also possible. These remarkable results opened a new therapeutic strategy against glioblastoma in targeting this molecular switch by transforming aggressive CSC in more indolent cells therefore accessible to classic therapies.
Thanks to a collaboration with Maria Duca, from the Nice Institute of Chemistry, a new molecule capable of acting on the switch is already identified and patented and will be tested a soon as possible in clinical trials.
Insets
An exemplary collaborative research
To fight against glioblastoma, Thierry Virolle organized a collaborative team between research staff from iBV and clinicians from several departments of Nice University Hospital.
Fanny Burel Vandenbos, Fabien Almairac and Philippe Paquis (director of the neurosurgery department) are also members of the team.
Fabien Almairac operates on patients and collects the tumors, Fanny Burel Vandenbos characterizes the samples. The tumor tissues are transmitted to the research laboratory and Laurent Turchi dissociates the tumors and puts the GSCs in culture. The scientific team is developing projects from this biological material.
Fanny and Fabien both did their science thesis at iBV in Thierry Virolle’s team and therefore participated in molecular studies. As an anatomopathologist, Fanny performs immunohistological markings on tumor sections and contributes to the interpretation of the results. Meetings between scientists and clinicians take place regularly to discuss the results and think on the conditions for transfer of these fundamental results to the clinic and to design clinical trial.
The toggle switch
The team has shown a very high plasticity of GBM cells, allowing them to pass from a differentiated state to a stem cell state and vice versa in function of environmental conditions, reproducing the important intra-tumor functional heterogeneity that exists in human GBMs. Moreover, they show that tumor cells exhibiting stem-like properties and expression of pluripotency markers, NANOG and OCT4, can arise from original differentiated tumor cells freshly isolated from human glioblastomas and which have never known any serum culture conditions.
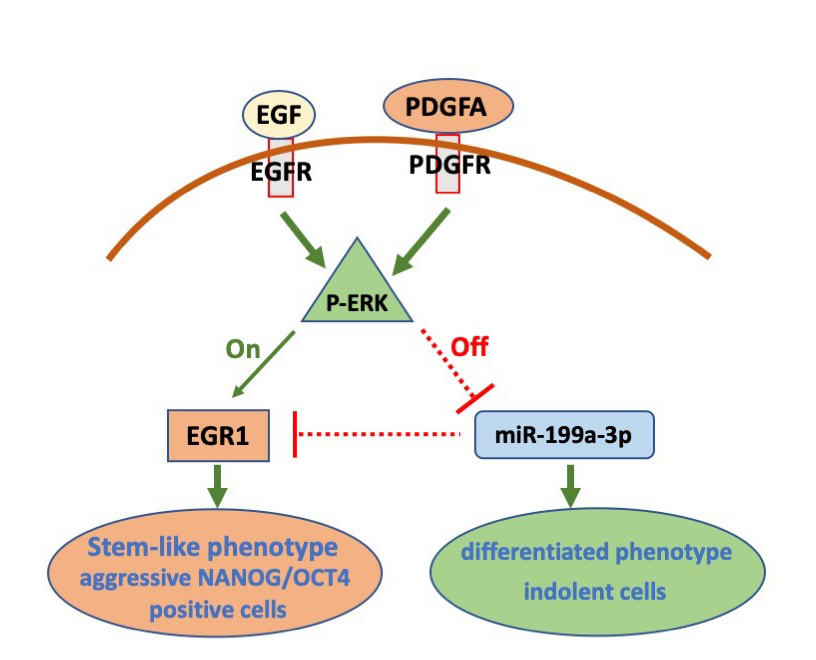
Switching from less aggressive, more differentiated cellular state to a self-renewing and strongly tumorigenic state, expressing NANOG and OCT4, is promoted by an EGFR/ERK mediated mechanism.
In differentiated GBM cells, ERK-mediated repression of miR-199a-3p induced EGR1 protein expression and triggered dedifferentiation. Overall, this signaling pathway constitutes an ERK-mediated “toggle switch” that promotes pluripotency marker expression and stem-like features in GBM cells.
For review
Figure
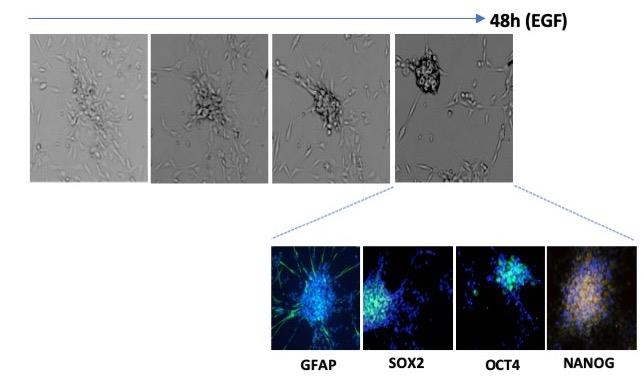
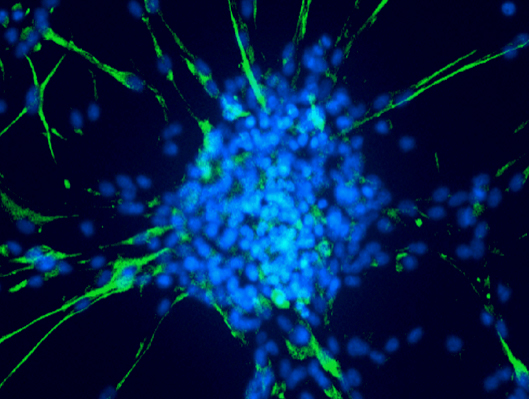
The adhering differentiated cells around the bud express the astrocyte differentiation marker GFAP, while the cancer stem cells in the center of the bud express it weakly but strongly express the stem cell markers SOX2, OCT4 and NANOG.