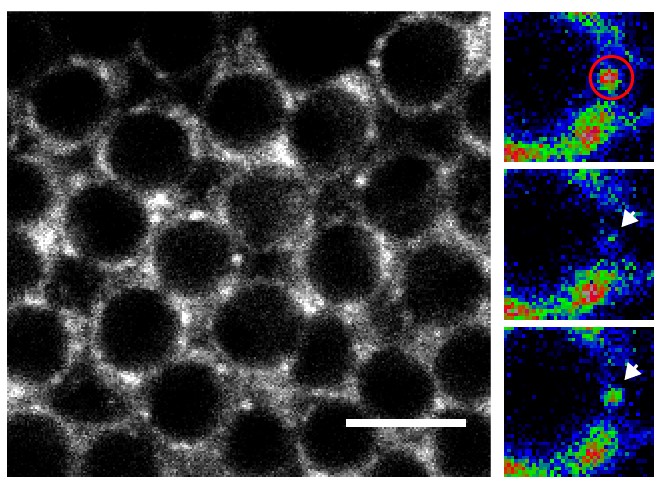
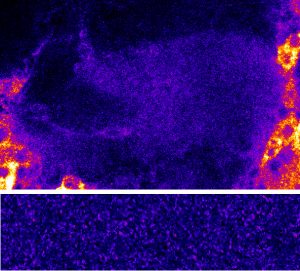
 The unstructured prion domains or Prion-like domains (PLDs) are present in hundreds of proteins. Although mutations in these domains have been shown to promote the development of neurodegenerative diseases, little is known about their physiological role. On the basis of a detailed functional analysis, Florence Besse and her team have revealed that the PLD of Drosophila Imp, a conserved component of neuronal ribonucleoprotein (RNP) granules, is essential for the developmentally-controlled localization of Imp RNP granules to axons and regulates in vivo axonal remodeling. These results published in the journal Nature communications uncover a physiological function for PLDs in the spatio-temporal control of neuronal RNP assemblies and open up new perspectives on the function of this long-ignored part of the proteome in healthy or pathological contexts.
The unstructured prion domains or Prion-like domains (PLDs) are present in hundreds of proteins. Although mutations in these domains have been shown to promote the development of neurodegenerative diseases, little is known about their physiological role. On the basis of a detailed functional analysis, Florence Besse and her team have revealed that the PLD of Drosophila Imp, a conserved component of neuronal ribonucleoprotein (RNP) granules, is essential for the developmentally-controlled localization of Imp RNP granules to axons and regulates in vivo axonal remodeling. These results published in the journal Nature communications uncover a physiological function for PLDs in the spatio-temporal control of neuronal RNP assemblies and open up new perspectives on the function of this long-ignored part of the proteome in healthy or pathological contexts.
Bibliography
The prion-like domain of Drosophila Imp promotes axonal transport of RNP granules in vivo.
Vijayakumar J, Perrois C, Heim M, Bousset L, Alberti S, Besse F. Florence Besse, Nat Commun. 2019 Jun 13.
https://www.nature.com/articles/s41467-019-10554-w
CNRS INSB press release
Contacts
CNRS Researcher | Florence Besse | T +33 4 89 15 07 45 | florence.besse@univ-cotedazur.fr